Sexually dimorphic oxytocin circuits drive intragroup social conflict and aggression in wild house mice
IF 21.2
1区 医学
Q1 NEUROSCIENCES
引用次数: 0
Abstract
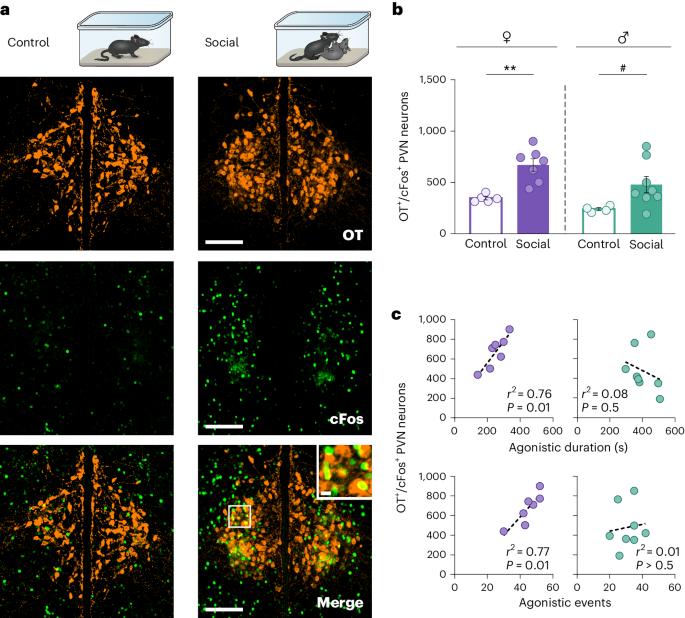
In nature, both males and females engage in competitive aggressive interactions to resolve social conflicts, yet the behavioral principles guiding such interactions and their underlying neural mechanisms remain poorly understood. Through circuit manipulations in wild mice, we unveil oxytocin-expressing (OT+) neurons in the hypothalamic paraventricular nucleus (PVN) as a neural hub governing behavior in dyadic and intragroup social conflicts, influencing the degree of behavioral sexual dimorphism. We demonstrate that OT+ PVN neurons are essential and sufficient in promoting aggression and dominance hierarchies, predominantly in females. Furthermore, pharmacogenetic activation of these neurons induces a change in the ‘personality’ traits of the mice within groups, in a sex-dependent manner. Finally, we identify an innervation from these OT neurons to the ventral tegmental area that drives dyadic aggression, in a sex-specific manner. Our data suggest that competitive aggression in naturalistic settings is mediated by a sexually dimorphic OT network connected with reward-related circuitry. In nature, female mice, like males, display aggression and dominant hierarchy. This study in wild mice identifies oxytocin-expressing neurons as a hub governing these behaviors, influencing the degree of sexual dimorphism in social conflicts.

性双态催产素回路驱动野生家鼠的群内社会冲突和攻击行为
在自然界中,雄性和雌性都会通过竞争性的攻击性互动来解决社会冲突,但人们对指导这种互动的行为原理及其潜在的神经机制仍然知之甚少。通过对野生小鼠进行回路操作,我们揭示了下丘脑室旁核(PVN)中表达催产素(OT+)的神经元是支配二人和群体内社会冲突行为的神经枢纽,影响行为的性别二态性程度。我们证明,OT+ PVN 神经元在促进攻击性和支配等级方面是必不可少和足够的,主要是在雌性中。此外,这些神经元的药物基因激活会诱导小鼠在群体中的 "个性 "特征发生变化,这种变化与性别有关。最后,我们确定了这些OT神经元向腹侧被盖区的神经传导,这种传导以性别特异性的方式驱动着二人攻击行为。我们的数据表明,自然环境中的竞争性攻击行为是由与奖赏相关电路相连的性双态 OT 网络介导的。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature neuroscience
医学-神经科学
CiteScore
38.60
自引率
1.20%
发文量
212
审稿时长
1 months
期刊介绍:
Nature Neuroscience, a multidisciplinary journal, publishes papers of the utmost quality and significance across all realms of neuroscience. The editors welcome contributions spanning molecular, cellular, systems, and cognitive neuroscience, along with psychophysics, computational modeling, and nervous system disorders. While no area is off-limits, studies offering fundamental insights into nervous system function receive priority.
The journal offers high visibility to both readers and authors, fostering interdisciplinary communication and accessibility to a broad audience. It maintains high standards of copy editing and production, rigorous peer review, rapid publication, and operates independently from academic societies and other vested interests.
In addition to primary research, Nature Neuroscience features news and views, reviews, editorials, commentaries, perspectives, book reviews, and correspondence, aiming to serve as the voice of the global neuroscience community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


