A streamlined method to generate endothelial cells from human pluripotent stem cells via transient doxycycline-inducible ETV2 activation
Abstract
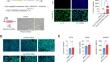
The development of reliable methods for producing functional endothelial cells (ECs) is crucial for progress in vascular biology and regenerative medicine. In this study, we present a streamlined and efficient methodology for the differentiation of human induced pluripotent stem cells (iPSCs) into induced ECs (iECs) that maintain the ability to undergo vasculogenesis in vitro and in vivo using a doxycycline-inducible system for the transient expression of the ETV2 transcription factor. This approach mitigates the limitations of direct transfection methods, such as mRNA-mediated differentiation, by simplifying the protocol and enhancing reproducibility across different stem cell lines. We detail the generation of iPSCs engineered for doxycycline-induced ETV2 expression and their subsequent differentiation into iECs, achieving over 90% efficiency within four days. Through both in vitro and in vivo assays, the functionality and phenotypic stability of the derived iECs were rigorously validated. Notably, these cells exhibit key endothelial markers and capabilities, including the formation of vascular networks in a microphysiological platform in vitro and in a subcutaneous mouse model. Furthermore, our results reveal a close transcriptional and proteomic alignment between the iECs generated via our method and primary ECs, confirming the biological relevance of the differentiated cells. The high efficiency and effectiveness of our induction methodology pave the way for broader application and accessibility of iPSC-derived ECs in scientific research, offering a valuable tool for investigating endothelial biology and for the development of EC-based therapies.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


