Evaluation of secretome biomarkers in glioblastoma cancer stem cells: A bioinformatics analysis
Abstract
Background
Glioblastoma (GBM) is a malignant brain tumor that frequently occurs alongside other central nervous system (CNS) conditions. The secretome of GBM cells contains a diverse array of proteins released into the extracellular space, influencing the tumor microenvironment. These proteins can serve as potential biomarkers for GBM due to their involvement in key biological processes, exploring the secretome biomarkers in GBM research represents a cutting-edge strategy with significant potential for advancing diagnostic precision, treatment monitoring, and ultimately improving outcomes for patients with this challenging brain cancer.
Aim
This study was aimed to investigate the roles of secretome biomarkers and their pathwayes in GBM through bioinformatics analysis.
Methods and Results
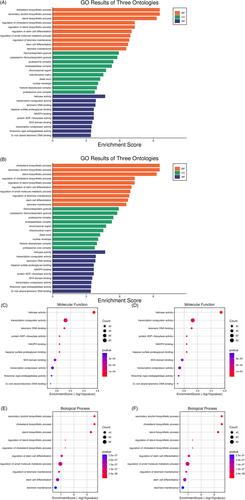
Using data from the Gene Expression Omnibus and the Cancer Genome Atlas datasets—where both healthy and cancerous samples were analyzed—we used a quantitative analytical framework to identify differentially expressed genes (DEGs) and cell signaling pathways that might be related to GBM. Then, we performed gene ontology studies and hub protein identifications to estimate the roles of these DEGs after finding disease-gene connection networks and signaling pathways. Using the GEPIA Proportional Hazard Model and the Kaplan–Meier estimator, we widened our analysis to identify the important genes that may play a role in both progression and the survival of patients with GBM. In total, 890 DEGs, including 475 and 415 upregulated and downregulated were identified, respectively. Our results revealed that SQLE, DHCR7, delta-1 phospholipase C (PLCD1), and MINPP1 genes are highly expressed, and the Enolase 2 (ENO2) and hexokinase-1 (HK1) genes are low expressions.
Conclusion
Hence, our findings suggest novel mechanisms that affect the occurrence of GBM development, growth, and/or establishment and may also serve as secretory biomarkers for GBM prognosis and possible targets for therapy. So, continued research in this field may uncover new avenues for therapeutic interventions and contribute to the ongoing efforts to combat GBM effectively.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


