One-Pot Synthesis of 4-Chloroquinolines via Bis(trichloromethyl) Carbonate and Triphenylphosphine Oxide-Mediated Cascade Reactions of N-Aryl Enaminones
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
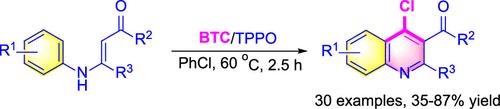
A novel method for synthesizing substituted 4-chloroquinolines has been devised, utilizing a cascade reaction of N-aryl enaminones promoted by bis(trichloromethyl) carbonate (BTC) and triphenylphosphine oxide (TPPO). This approach features accessible starting materials, a broad substrate range, extensive functional group compatibility, gentle reaction conditions, and straightforward operation. Its versatility is evidenced by its facile scalability and suitability for late-stage derivatization. A plausible mechanism involving α-carbonylation, 6π-azaelectrocyclization, and dehydroxychlorination sequence is proposed.
通过碳酸二(三氯甲基)酯和三苯基膦氧化物介导的 N-芳基烯丙基胺的级联反应一锅合成 4-氯喹啉。
利用碳酸二(三氯甲基)酯(BTC)和氧化三苯基膦(TPPO)促进的 N-芳基烯酮级联反应,设计出了一种合成取代的 4-氯喹啉的新方法。这种方法的特点是起始材料易得、底物范围广、官能团兼容性强、反应条件温和、操作简单。这种方法的多功能性体现在其易于扩展和适合后期衍生。提出了一种涉及α-羰基化、6π-氮电环化和脱羟基氯化顺序的合理机制。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


