Structure elucidation of plumerubradins A–C: Correlations between 1H NMR signal patterns and structural information of [2+2]-type cyclobutane derivatives
IF 9.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
[2+2]-Type cyclobutane derivatives comprise a large family of natural products with diverse molecular architectures. However, the structure elucidation of the cyclobutane ring, including its connection mode and stereochemistry, presents a significant challenge. Plumerubradins A–C (–), three novel iridoid glycoside [2+2] dimers featuring a highly functionalized cyclobutane core and multiple stereogenic centers, were isolated from the flowers of Plumeria rubra. Through biomimetic semisynthesis and chemical degradation of compounds –, synthesis of phenylpropanoid-derived [2+2] dimers –, combined with extensive spectroscopic analysis, single-crystal X-ray crystallography, and microcrystal electron diffraction experiments, the structures with absolute configurations of – were unequivocally elucidated. Furthermore, quantum mechanics-based H NMR iterative full spin analysis successfully established the correlations between the signal patterns of cyclobutane protons and the structural information of the cyclobutane ring in phenylpropanoid-derived [2+2] dimers, providing a diagnostic tool for the rapid structural elucidation of [2+2]-type cyclobutane derivatives.
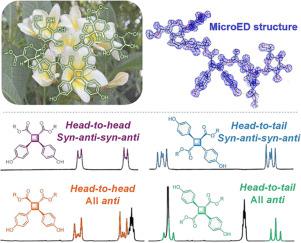
plumerubradins A-C 的结构阐释:[2+2]型环丁烷衍生物的 1H NMR 信号模式与结构信息之间的相关性
[2+2]型环丁烷衍生物是一个庞大的天然产物家族,其分子结构多种多样。然而,环丁烷环的结构阐明,包括其连接方式和立体化学,是一项重大挑战。Plumerubradins A-C (-) 是三种新型鸢尾甙 [2+2] 二聚体,具有高度官能化的环丁烷核心和多个立体中心。通过对化合物-的生物仿生半合成和化学降解、苯丙苷衍生[2+2]二聚体-的合成,并结合大量光谱分析、单晶 X 射线晶体学和微晶电子衍射实验,明确阐明了-的绝对构型结构。此外,基于量子力学的氢核磁共振迭代全自旋分析成功地建立了环丁烷质子信号模式与苯丙类衍生[2+2]二聚体中环丁烷环结构信息之间的相关性,为快速阐明[2+2]型环丁烷衍生物的结构提供了诊断工具。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chinese Chemical Letters
化学-化学综合
CiteScore
14.10
自引率
15.40%
发文量
8969
审稿时长
1.6 months
期刊介绍:
Chinese Chemical Letters (CCL) (ISSN 1001-8417) was founded in July 1990. The journal publishes preliminary accounts in the whole field of chemistry, including inorganic chemistry, organic chemistry, analytical chemistry, physical chemistry, polymer chemistry, applied chemistry, etc.Chinese Chemical Letters does not accept articles previously published or scheduled to be published. To verify originality, your article may be checked by the originality detection service CrossCheck.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


