Striatal projection neurons coexpressing dopamine D1 and D2 receptors modulate the motor function of D1- and D2-SPNs
IF 21.2
1区 医学
Q1 NEUROSCIENCES
引用次数: 0
Abstract
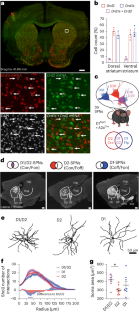
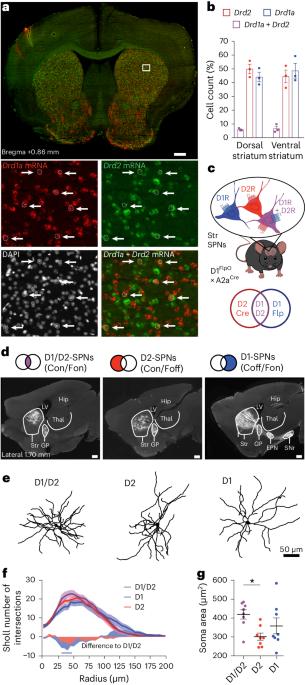
The role of the striatum in motor control is commonly assumed to be mediated by the two striatal efferent pathways characterized by striatal projection neurons (SPNs) expressing dopamine (DA) D1 receptors or D2 receptors (D1-SPNs and D2-SPNs, respectively), without regard to SPNs coexpressing both receptors (D1/D2-SPNs). Here we developed an approach to target these hybrid SPNs in mice and demonstrate that, although these SPNs are less abundant, they have a major role in guiding the motor function of the other two populations. D1/D2-SPNs project exclusively to the external globus pallidus and have specific electrophysiological features with distinctive integration of DA signals. Gain- and loss-of-function experiments indicate that D1/D2-SPNs potentiate the prokinetic and antikinetic functions of D1-SPNs and D2-SPNs, respectively, and restrain the integrated motor response to psychostimulants. Overall, our findings demonstrate the essential role of this population of D1/D2-coexpressing neurons in orchestrating the fine-tuning of DA regulation in thalamo-cortico-striatal loops. Bonnavion, Varin and colleagues show that striatal projection neurons that coexpress dopamine D1 and D2 receptors have unique physiological properties and serve as a crucial third output in the striatum for motor control and dopaminergic signal integration.
共表达多巴胺 D1 和 D2 受体的纹状体投射神经元调节 D1 和 D2-SPN 的运动功能
纹状体在运动控制中的作用通常被认为是由纹状体突起神经元(SPNs)表达多巴胺(DA)D1受体或D2受体(分别为D1-SPNs和D2-SPNs)的两条纹状体传出通路介导的,而不考虑同时表达两种受体的SPNs(D1/D2-SPNs)。在这里,我们开发了一种以小鼠中这些混合 SPN 为靶点的方法,并证明尽管这些 SPN 的数量较少,但它们在指导其他两个种群的运动功能方面发挥着重要作用。D1/D2-SPNs只投射到苍白球外侧,具有特殊的电生理特征,能对DA信号进行独特的整合。功能增益和功能缺失实验表明,D1/D2-SPNs 可分别增强 D1-SPNs 和 D2-SPNs 的促运动和抗运动功能,并抑制对精神兴奋剂的综合运动反应。总之,我们的研究结果表明,在丘脑-皮质-纹状体环路中,D1/D2共表达神经元群在协调DA调节的微调过程中起着至关重要的作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature neuroscience
医学-神经科学
CiteScore
38.60
自引率
1.20%
发文量
212
审稿时长
1 months
期刊介绍:
Nature Neuroscience, a multidisciplinary journal, publishes papers of the utmost quality and significance across all realms of neuroscience. The editors welcome contributions spanning molecular, cellular, systems, and cognitive neuroscience, along with psychophysics, computational modeling, and nervous system disorders. While no area is off-limits, studies offering fundamental insights into nervous system function receive priority.
The journal offers high visibility to both readers and authors, fostering interdisciplinary communication and accessibility to a broad audience. It maintains high standards of copy editing and production, rigorous peer review, rapid publication, and operates independently from academic societies and other vested interests.
In addition to primary research, Nature Neuroscience features news and views, reviews, editorials, commentaries, perspectives, book reviews, and correspondence, aiming to serve as the voice of the global neuroscience community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


