Direct observation of translational activation by a ribonucleoprotein granule
IF 17.3
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
Abstract
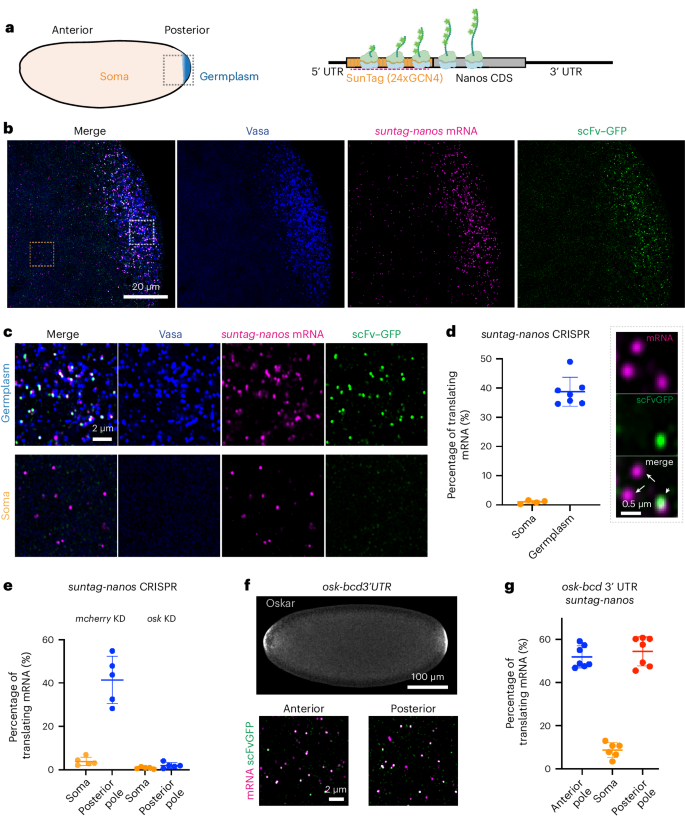
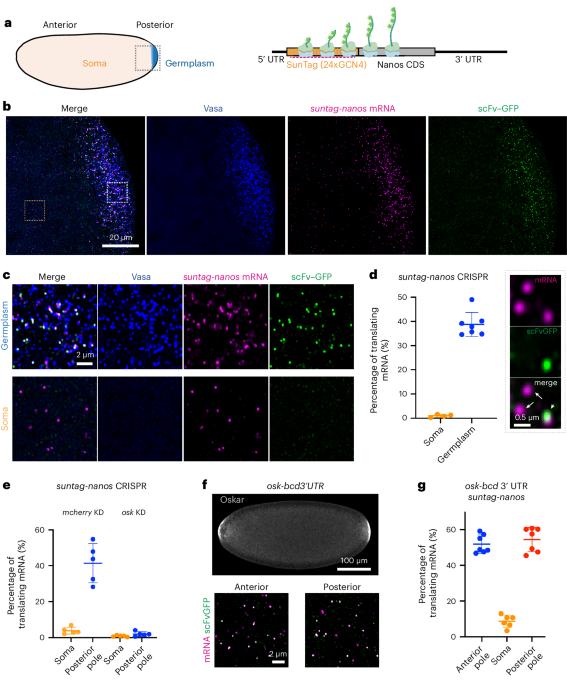
Biomolecular condensates organize biochemical processes at the subcellular level and can provide spatiotemporal regulation within a cell. Among these, ribonucleoprotein (RNP) granules are storage hubs for translationally repressed mRNA. Whether RNP granules can also activate translation and how this could be achieved remains unclear. Here, using single-molecule imaging, we demonstrate that the germ cell-determining RNP granules in Drosophila embryos are sites for active translation of nanos mRNA. Nanos translation occurs preferentially at the germ granule surface with the 3′ UTR buried within the granule. Smaug, a cytosolic RNA-binding protein, represses nanos translation, which is relieved when Smaug is sequestered to the germ granule by the scaffold protein Oskar. Together, our findings uncover a molecular process by which RNP granules achieve localized protein synthesis through the compartmentalized loss of translational repression. Chen et al. perform single-molecule imaging of translation at ribonucleoprotein (RNP) granules. They show that RNP granule surfaces are sites of nanos mRNA translation, whereas the granule interior is translationally repressive.
直接观察核糖核蛋白颗粒的翻译激活过程
生物分子凝聚体在亚细胞水平上组织生化过程,并能在细胞内提供时空调控。其中,核糖核蛋白(RNP)颗粒是被翻译抑制的 mRNA 的储存中心。RNP 颗粒是否也能激活翻译以及如何实现这一点仍不清楚。在这里,我们利用单分子成像技术证明,果蝇胚胎中决定生殖细胞的 RNP 颗粒是 nanos mRNA 活跃翻译的场所。Nanos 的翻译优先发生在生殖细胞颗粒表面,而 3′ UTR 则埋藏在颗粒内。Smaug是一种细胞质RNA结合蛋白,它能抑制nanos的翻译,当Smaug被支架蛋白Oskar封闭在胚芽颗粒中时,这种抑制作用就会被解除。总之,我们的研究结果揭示了一个分子过程,RNP 颗粒通过分区失去翻译抑制作用来实现局部蛋白质合成。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
Nature Cell Biology
生物-细胞生物学
CiteScore
28.40
自引率
0.90%
发文量
219
审稿时长
3 months
期刊介绍:
Nature Cell Biology, a prestigious journal, upholds a commitment to publishing papers of the highest quality across all areas of cell biology, with a particular focus on elucidating mechanisms underlying fundamental cell biological processes. The journal's broad scope encompasses various areas of interest, including but not limited to:
-Autophagy
-Cancer biology
-Cell adhesion and migration
-Cell cycle and growth
-Cell death
-Chromatin and epigenetics
-Cytoskeletal dynamics
-Developmental biology
-DNA replication and repair
-Mechanisms of human disease
-Mechanobiology
-Membrane traffic and dynamics
-Metabolism
-Nuclear organization and dynamics
-Organelle biology
-Proteolysis and quality control
-RNA biology
-Signal transduction
-Stem cell biology
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


