Brain–body mechanisms contribute to sexual dimorphism in amyotrophic lateral sclerosis
IF 28.2
1区 医学
Q1 CLINICAL NEUROLOGY
引用次数: 0
Abstract
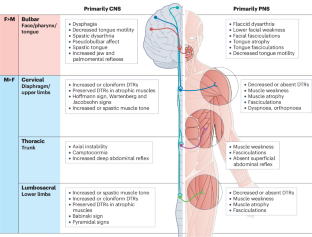
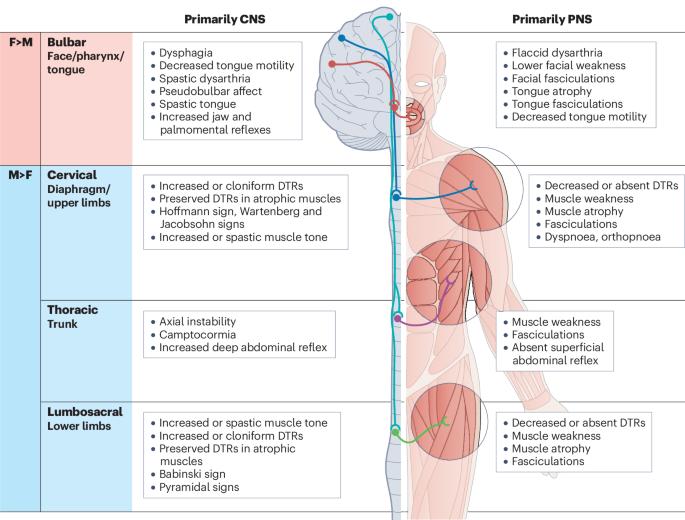
Amyotrophic lateral sclerosis (ALS) is the most common form of human motor neuron disease. It is characterized by the progressive degeneration of upper and lower motor neurons, leading to generalized motor weakness and, ultimately, respiratory paralysis and death within 3–5 years. The disease is shaped by genetics, age, sex and environmental stressors, but no cure or routine biomarkers exist for the disease. Male individuals have a higher propensity to develop ALS, and a different manifestation of the disease phenotype, than female individuals. However, the mechanisms underlying these sex differences remain a mystery. In this Review, we summarize the epidemiology of ALS, examine the sexually dimorphic presentation of the disease and highlight the genetic variants and molecular pathways that might contribute to sex differences in humans and animal models of ALS. We advance the idea that sexual dimorphism in ALS arises from the interactions between the CNS and peripheral organs, involving vascular, metabolic, endocrine, musculoskeletal and immune systems, which are strikingly different between male and female individuals. Finally, we review the response to treatments in ALS and discuss the potential to implement future personalized therapeutic strategies for the disease. Amyotrophic lateral sclerosis (ALS) differs considerably in prevalence and manifestation between sexes. This Review summarizes sexual dimorphism in the epidemiology, clinical presentation and disease mechanisms of ALS and explores the role of brain–body interactions in driving sex-dependent pathogenesis.
脑-体机制导致肌萎缩性脊髓侧索硬化症的性双态性
肌萎缩侧索硬化症(ALS)是人类运动神经元疾病中最常见的一种。其特征是上下运动神经元逐渐退化,导致全身运动无力,最终导致呼吸麻痹,并在 3-5 年内死亡。这种疾病受遗传、年龄、性别和环境压力等因素的影响,但目前还没有治愈这种疾病的方法或常规生物标志物。与女性相比,男性更容易患上渐冻人症,其疾病表型的表现也与女性不同。然而,这些性别差异的内在机制仍是一个谜。在这篇综述中,我们总结了 ALS 的流行病学,研究了该疾病的性别二形表现,并强调了可能导致人类和 ALS 动物模型性别差异的基因变异和分子途径。我们提出的观点是,肌萎缩性脊髓侧索硬化症的性别双态性源于中枢神经系统和外周器官之间的相互作用,涉及血管、代谢、内分泌、肌肉骨骼和免疫系统,这些系统在男性和女性个体之间存在显著差异。最后,我们回顾了 ALS 的治疗反应,并讨论了未来对该疾病实施个性化治疗策略的可能性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Reviews Neurology
医学-临床神经学
CiteScore
29.90
自引率
0.80%
发文量
138
审稿时长
6-12 weeks
期刊介绍:
Nature Reviews Neurology aims to be the premier source of reviews and commentaries for the scientific and clinical communities we serve. We want to provide an unparalleled service to authors, referees, and readers, and we work hard to maximize the usefulness and impact of each article. The journal publishes Research Highlights, Comments, News & Views, Reviews, Consensus Statements, and Perspectives relevant to researchers and clinicians working in the field of neurology. Our broad scope ensures that the work we publish reaches the widest possible audience. Our articles are authoritative, accessible, and enhanced with clearly understandable figures, tables, and other display items. This page gives more detail about the aims and scope of the journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


