BSACI guidance for the implementation of Palforzia® peanut oral immunotherapy in the United Kingdom: A Delphi consensus study
Abstract
Background
Palforzia® enables the safe and effective desensitisation of children with peanut allergy. The treatment pathway requires multiple visits for dose escalation, up-dosing, monitoring of patients taking maintenance therapy and conversion onto daily real-world peanut consumption. The demand for peanut immunotherapy outstrips current National Health Service (NHS) capacity and requires services to develop a national consensus on how best to offer Palforzia® in a safe and equitable manner. We undertook a Delphi consensus exercise to determine guidance statements for the implementation of Palforzia®-based immunotherapy in the NHS.
Methods
We undertook focus groups with children and young people who had received peanut immunotherapy to assess what was important for them and their carers. Common themes from patients formed the basis of creating draft statements. A panel of 18 multi-disciplinary professionals engaged in two rounds of anonymised voting to adapt the statements and score their importance. A final consensus workshop consolidated any variation in comments and scores to develop the final guidance statements.
Results
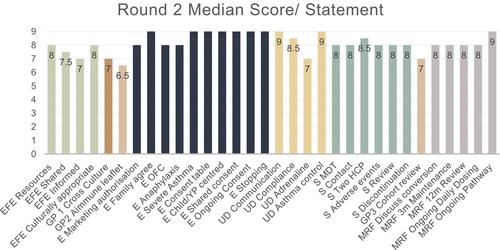
The panel achieved consensus on 91% (29/32) of guidance statements, demonstrating strong consensus around pragmatic principles for assuring the integrity of consent, safety and conversion from Palforzia® to real-world peanut products. The greatest amount of feedback was generated from three broad issues; (i) whether eligibility assessment should include compulsory peanut challenges and whether these should be designed to assess the threshold at which patients react to peanut, (ii) the governance processes to best ensure that patients' interests are prioritised and (iii) how to safely transition young people to other services, or discharge them, while they are taking daily peanut.
Conclusions
This consensus highlights the urgent need for the NHS to increase capacity for undertaking diagnostic food challenges as well as developing Palforzia® immunotherapy pathways. The voting panel agreed that families of peanut allergic children should be made aware of immunotherapy, that eligibility assessment should include how co-morbid conditions are managed and that services should monitor for adverse effects. The finalised statements are now published online for clinical practice in the UK. These guidance statements will be adapted in the coming years as more evidence is published and as the international experience of peanut immunotherapy evolves.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


