Harvesting surface (interfacial) energy for tribocatalytic degradation of hazardous dye pollutants using nanostructured materials: A review
Abstract
Introduction
Tribocatalysis, an emerging cutting-edge technique that uses frictional mechanical energy to activate the catalytic operation of a reaction or material including nanomaterials has garnered the interest of the research community in recent times.
Aim
This study aimed to critically review original research works directed toward tribocatalytic degradation of various hazardous dye pollutants. Notably, in this review, various nanomaterials and their composites with outstanding tailored degradation profiles are explored for their tribocatalytic degradation efficiency for various dye pollutants. In addition, the effect of various operating factors that are of importance to engineers, industries, and investors for optimization purposes was pragmatically discussed. Also, the effect of electron trapping and radical scavengers alongside the mechanism of tribocatalytic degradation was empirically analyzed.
Results
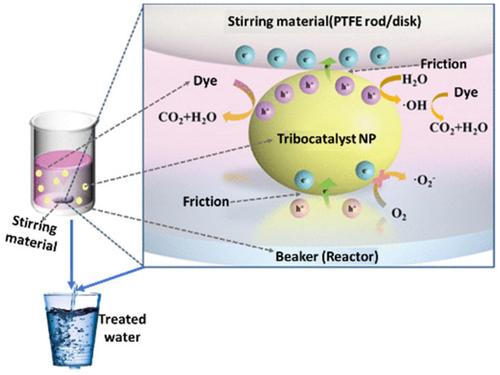
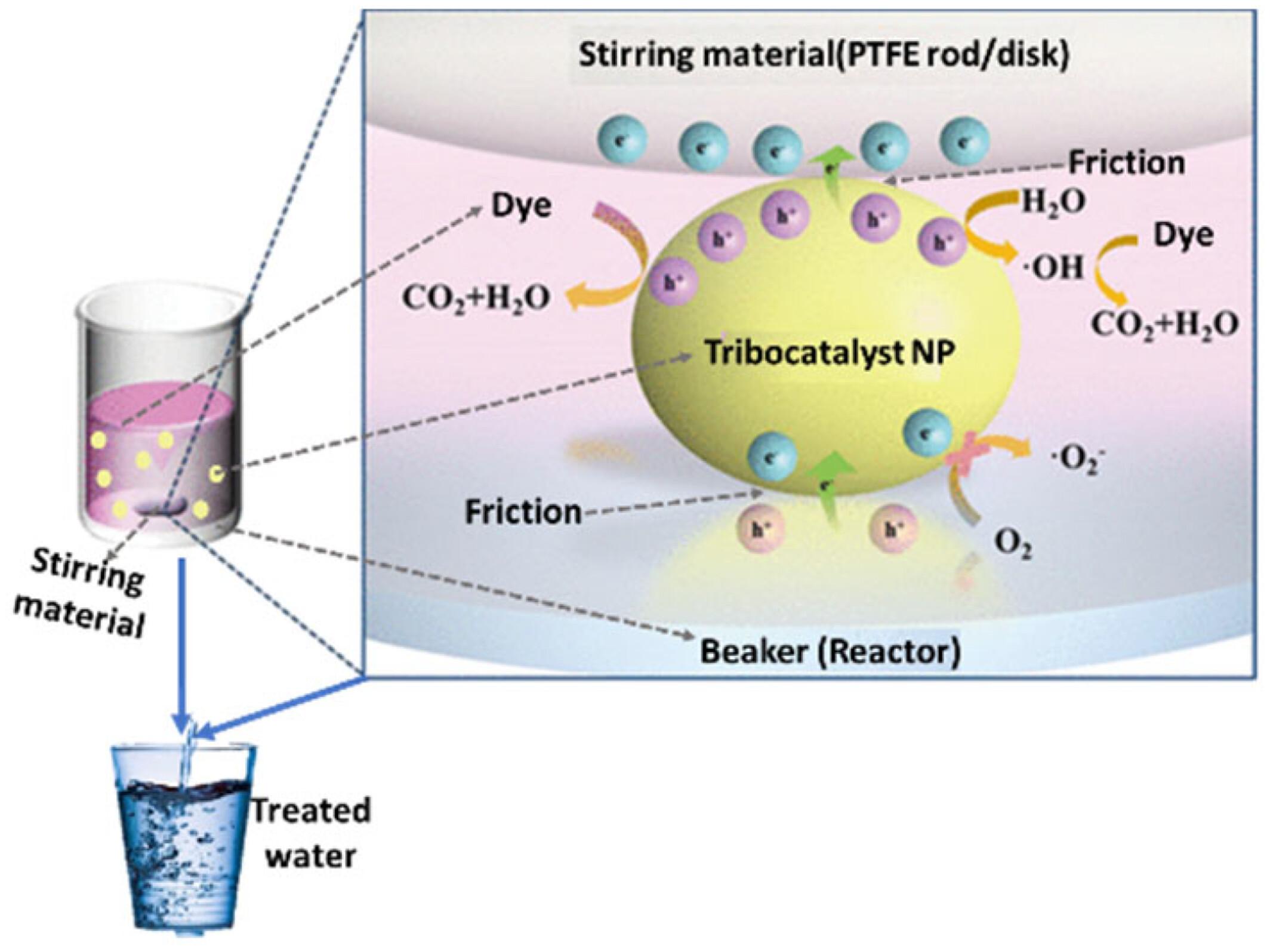
From this work, it was found that the maximum tribocatalytic degradation efficiency was >80% in most cases at an optimum temperature of 20–40°C, time taken of 0.5-48 hours, and stirring speed of 500-1000rmp. It was discovered that magnetic stirring enhances the production of •OH, O2•, and h+ by the nanomaterials that are mechanistically responsible for the degradation of the dye pollutants. Also, it was revealed that expended tribocatalyst can be eluted mostly using H2O and can be reused up to 3–10 times while still sustaining degradation efficiency of >80% in most cases and this suggests the industrial scalability and eco-friendliness potential of this approach.
Conclusion
In the end, challenges and research gaps that can pave the way for method improvement and also serve as future research hotspots for researchers were presented.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


