Cyclolignan synthesis streamlined by enantioselective hydrogenation of tetrasubstituted olefins
0 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
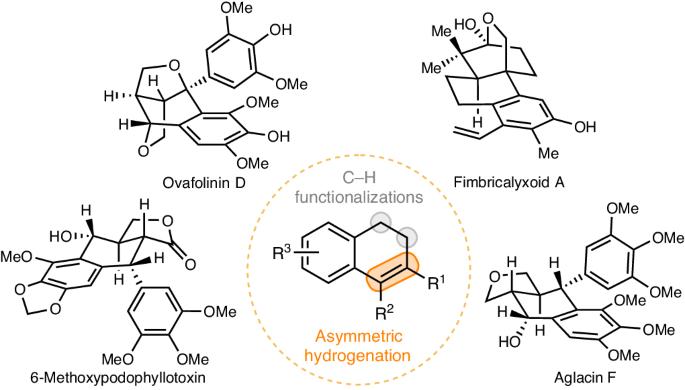
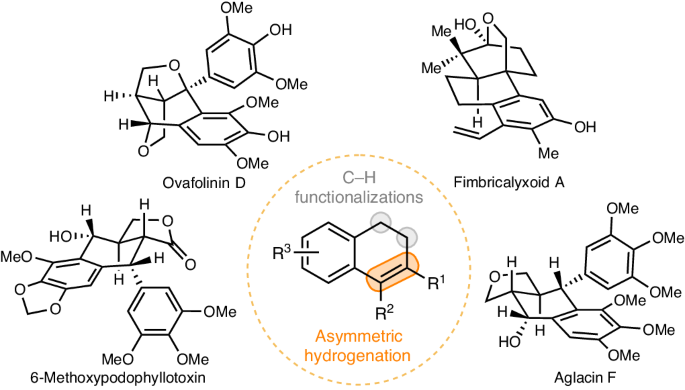
Natural products have long been valuable sources of inspiration for drug discovery. Unfortunately, the inherent limitations of direct semisynthetic derivatizations have become clear, and the need to overcome these limitations is now particularly urgent because the most valuable natural products tend to be isolated in minute amounts; de novo synthesis with ideal modularity and diversity is therefore a critical goal in drug discovery research. Herein we report a powerful, general platform for cyclolignan synthesis that involves challenging rhodium-catalysed enantioselective hydrogenation of tetrasubstituted 1,2-dihydronaphthalene esters (>40 examples; up to 99% yield, >99% e.e.). This unique platform allows ready access to various types of cyclolignans, as exemplified by the expedient and mostly protecting-group-free synthesis of over thirty cyclolignans, including many that have not previously been synthesized, such as 6-methoxy podophyllotoxin, cleistantoxin, picrobursenin, austrobailignan-4, (+)-lirionol, (+)-gaultherin C, ovafolinin D, fimbricalyxoid A, and aglacins D, F–H (with three revised structures). We expect this work to inspire modular, de novo syntheses of other important classes of natural products and thus to rejuvenate the role of natural products in drug discovery and development. Semisynthetic derivatization approaches to synthesize cyclolignans are limited to using natural sources with inflexible structural features. Now, a powerful, general platform for the synthesis of various types of optically active cyclolignans is achieved through the strategic application of the asymmetric hydrogenation of tetrasubstituted olefins and C(sp3)–H functionalization.
通过四取代烯烃的对映选择性氢化简化环木质素的合成
长期以来,天然产物一直是药物发现的宝贵灵感来源。不幸的是,直接半合成衍生化的固有局限性已经非常明显,而克服这些局限性的需求现在尤为迫切,因为最有价值的天然产物往往只能分离到极少量;因此,具有理想的模块化和多样性的从头合成是药物发现研究的关键目标。在本文中,我们报告了一个功能强大的环木质素合成通用平台,该平台涉及铑催化的四取代 1,2-二氢萘酯的对映选择性氢化(40 个实例;收率高达 99%,等效度为 99%)。这种独特的平台可以随时获得各种类型的环木质素,例如,可以方便地合成三十多种环木质素,而且大多不需要保护基,其中包括许多以前从未合成过的环木质素、例如 6-甲氧基荚叶毒素、cleistantoxin、picrobursenin、austrobailignan-4、(+)-lirionol、(+)-gaultherin C、ovafolinin D、fimbricalyxoid A 和 aglacins D、F-H(具有三个修订结构)。我们期待这项工作能激发对其他重要类别天然产物的模块化从头合成,从而使天然产物在药物发现和开发中的作用焕发新的活力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


