O-to-O acyl transfer for epimerization-free peptide C-terminal salicylaldehyde ester synthesis
0 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
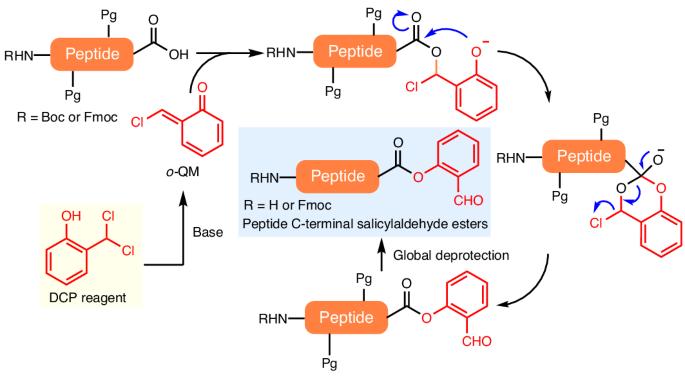
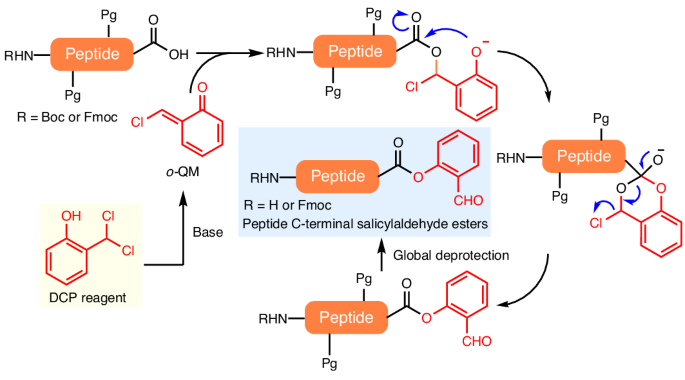
Peptide salicylaldehyde esters are the requisite coupling partner in Ser/Thr ligation reactions towards chemical protein synthesis. In general, it would be cost-effective and efficient to use side-chain-protected peptide acids, after Fmoc solid-phase peptide synthesis, for direct C-terminal derivatization; however, this has yet to be achieved, due to an intrinsic epimerization pathway. Here we report the development of 2-(dichloromethyl)phenol as a reagent that can directly form peptide salicylaldehyde esters in an epimerization-free manner. Mechanistic studies reveal that the 2-(dichloromethyl)phenol reagent serves as a source of highly reactive quinone methide species that can be trapped by the peptide C-terminal carboxylate to give α-chloroesters, followed by an Obenzylic-to-Ophenolic acyl transfer and chloride extrusion process. The peptide salicylaldehyde ester reaction products have been applied in the convergent total chemical synthesis of linker histone H1.2 using sequential Ser/Thr ligation reactions. C-terminal peptide salicylaldehyde ester synthesis is a challenge due to intrinsic epimerization. Now the development of epimerization-free synthesis of peptide C-terminal salicylaldehyde esters is reported. The approach uses side-chain-protected peptides, formed through solid-phase synthesis, and 2-(dichloromethyl)phenol as substrates and proceeds through an O-to-O acyl transfer process.
用于无表聚肽 C 端水杨醛酯合成的 O 到 O酰基转移
肽水杨醛酯是 Ser/Thr 连接反应中必要的偶联剂,可用于化学蛋白质合成。一般来说,在 Fmoc 固相肽合成后,使用侧链保护的肽酸直接进行 C 端衍生是既经济又高效的方法;然而,由于其固有的外嵌合途径,这种方法尚未实现。在此,我们报告了 2-(二氯甲基)苯酚作为一种试剂的开发情况,它能以无表聚的方式直接形成肽水杨醛酯。机理研究表明,2-(二氯甲基)苯酚试剂是高活性醌类甲酰胺的来源,可被肽的 C 端羧酸酯捕获,生成 α-氯酯,然后进行苄基-酚酰基转移和氯挤出过程。肽水杨醛酯反应产物已被应用于连接子组蛋白 H1.2 的聚合全化学合成中,使用的是连续的 Ser/Thr 连接反应。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


