Boryl radical-mediated halogen-atom transfer enables arylation of alkyl halides with electrophilic and nucleophilic coupling partners
0 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
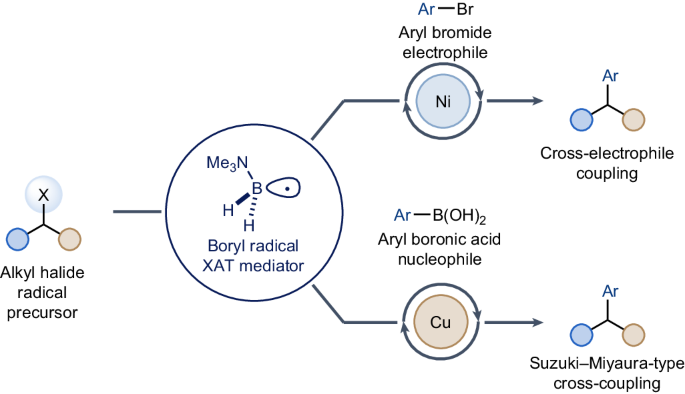
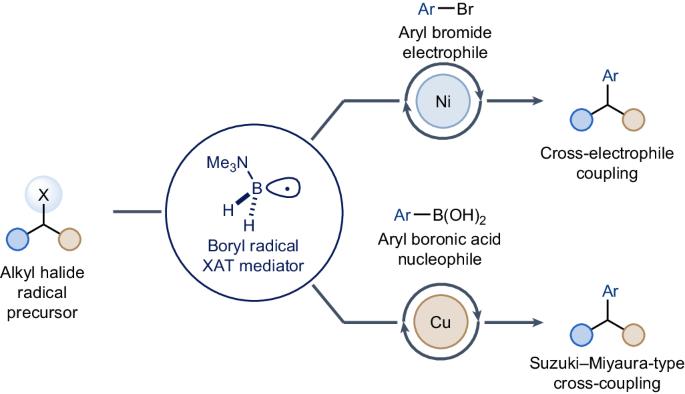
Traditional metal-catalysed cross-couplings of alkyl halides for C(sp3)–C(sp2) bond formation are often challenging to achieve. Processes where the alkyl halide is initially converted into a radical species can provide valuable complementarity. So far, these strategies are almost exclusively orchestrated by silicon-based reagents, which can be expensive, have low atom economy and are sensitive to steric factors. Here we report the use of the stable Lewis acid–Lewis base complex Me3N–BH3, which, upon conversion into its corresponding amine-ligated boryl radical, enables nickel- and copper-catalysed cross-coupling of alkyl iodides and bromides with electrophilic aryl bromides and nucleophilic aryl boronic acids. Mechanistically, this method uses the amine borane radical’s propensity to activate halides via halogen-atom transfer through highly polarized transition states. This reactivity features mild conditions and broad tolerability of functional groups and engages sterically hindered alkyl halides. The use of alkyl halides in radical cross-couplings generally requires silicon reagents as halogen abstractors. Now Me3N–BH3 is reported to facilitate these couplings with both aryl bromides and aryl boronic acids under either nickel or copper catalysis.
硼烷基自由基介导的卤素原子转移使烷基卤化物与亲电和亲核偶联伙伴发生芳基化反应
传统的金属催化烷基卤化物交叉耦合以形成 C(sp3)-C(sp2)键往往难以实现。烷基卤最初转化为自由基的过程可以提供宝贵的互补性。迄今为止,这些策略几乎都是通过硅基试剂来实现的,硅基试剂价格昂贵、原子经济性低,而且对立体因素很敏感。在这里,我们报告了使用稳定的路易斯酸-路易斯碱复合物 Me3N-BH3,在将其转化为相应的胺配位硼酸基后,可实现镍和铜催化的烷基碘化物和溴化物与亲电芳基溴化物和亲核芳基硼酸的交叉偶联。从机理上讲,这种方法利用了胺硼烷自由基通过高度极化的过渡态进行卤原子转移来激活卤化物的倾向。这种反应性的特点是条件温和,对官能团和受立体阻碍的烷基卤化物具有广泛的耐受性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


