Chemoenzymatic synthesis of macrocycles via dynamic kinetic resolution of secondary alcohols
IF 20
0 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
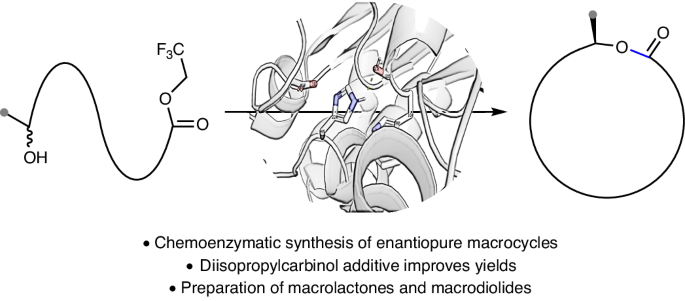
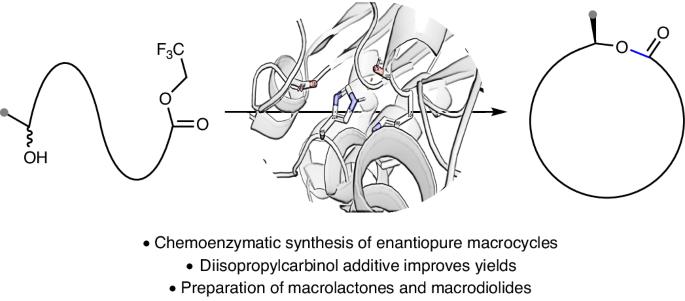
Macrolactones are privileged motifs in materials science, aromachemicals and pharmaceuticals. The pivotal ester linkage is often formed from chiral secondary alcohols, with macrolactonization using stoichiometric reagents to ensure retention or inversion of stereochemistry without compromising enantiopurity. An ideal strategy for macrolactonization is via dynamic kinetic resolution (DKR), which involves the simultaneous formation of the ester bond and introduction of a chiral centre with high stereocontrol. Surprisingly, a DKR method within the context of macrocyclization is yet to be reported. Here, using a chemoenzymatic approach, the macrocyclic DKR of seco esters affords enantioenriched macrolactones. An optimized protocol (using Candida antarctica lipase B (~0.04 mol%) and Shvo’s catalyst) forms 14–19-membered macrocycles with excellent enantioselectivities (85–99% e.e.). A variety of macrolactones were synthesized including aliphatic macrocycles, meta- and paracyclophanes as well as a macrodiolide via a dimerization protocol that was converted to the natural product macrolide (−)-pyrenophorin. The dynamic kinetic resolution of secondary alcohols for the synthesis of macrocycles is reported. This approach uses a chemoenzymatic method to form enantioenriched 14–19-membered macrolactones and macrodiolides and can be used to prepare bioactive natural products.
通过仲醇的动态动力学解析化学合成大环
大内酯是材料科学、芳香化学品和医药领域的重要主题。关键的酯连接通常是由手性仲醇形成的,大内酯化时要使用定量试剂,以确保在不影响对映体纯度的情况下保留或反转立体化学。大内酯化的理想策略是通过动态动力学解析(DKR),即同时形成酯键和引入具有高度立体控制的手性中心。令人惊讶的是,大环化背景下的 DKR 方法尚未见报道。在这里,利用化学酶法,仲酯的大环 DKR 生成了对映体富集的大内酯。优化方案(使用白色念珠菌脂肪酶 B(约 0.04 摩尔%)和 Shvo 催化剂)可形成 14-19 元的大环,对映选择性极佳(85-99% e.e.)。通过二聚化协议合成了多种大内酯,包括脂肪族大环、元环和对位环以及一种大环二醇,并将其转化为天然产物大环内酯 (-)-pyrenophorin 。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


