Oleic and linoleic acids induce oxidative stress in chondrocytes by inhibiting autophagy-regulated ciliogenesis
IF 8.5
4区 医学
Q1 MATERIALS SCIENCE, BIOMATERIALS
引用次数: 0
Abstract
The lack of a cure for osteoarthritis (OA), a prevalent joint disease among older individuals, remains an ongoing challenge. Obesity is a common risk factor for OA. Chondrocyte autophagy plays a crucial role in delaying the onset of OA. Our previous studies have demonstrated a significant elevation in the levels of oleic acid (OLA) and linoleic acid (LA) in the synovial fluid (SF) of patients with OA and obesity compared to those with OA alone, and an inhibitory effect of these molecules on the activation of autophagy. Accumulating evidence indicates a reciprocal regulatory relationship between autophagy and ciliogenesis; however, whether autophagy-mediated ciliogenesis plays a significant role in the pathogenesis of OA remains unclear. In this study, we aimed to determine whether OLA and LA affect OA development via the regulation of chondrocyte autophagy-mediated ciliogenesis. We found that both molecules inhibited this process in chondrocytes. Moreover, intracellular calcium and reactive oxygen species (ROS) levels increased simultaneously. Further, we explored the relationship between autophagy and ciliogenesis in chondrocytes. Activation of autophagy by rapamycin significantly attenuated the ciliogenesis inhibition caused by OLA and LA. Importantly, the downregulation of AKT and mTOR expression in chondrocytes reversed the autophagy-mediated inhibition of ciliogenesis and the ROS-accumulation-mediated inflammation induced by OLA and LA. Taken together, our results suggest that OLA and LA induce calcium-overload-driven ROS accumulation via autophagy-mediated ciliogenic disorders during OA pathogenesis. These findings demonstrate that targeting autophagy and ciliogenesis in chondrocytes is a protective strategy in the OA pathogenesis induced by OLA and LA.
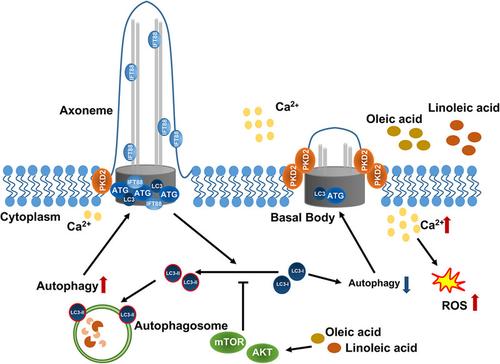
油酸和亚油酸通过抑制自噬调控的纤毛生成诱导软骨细胞氧化应激
骨关节炎(OA)是老年人中普遍存在的一种关节疾病,其治疗方法的缺乏仍是一个持续的挑战。肥胖是导致 OA 的常见风险因素。软骨细胞自噬在延缓 OA 发病方面起着至关重要的作用。我们之前的研究表明,与单纯的 OA 患者相比,OA 和肥胖患者滑液中的油酸(OLA)和亚油酸(LA)水平明显升高,而且这些分子对自噬的激活有抑制作用。越来越多的证据表明,自噬和纤毛生成之间存在相互调控的关系;然而,自噬介导的纤毛生成是否在 OA 的发病机制中发挥重要作用仍不清楚。在本研究中,我们旨在确定OLA和LA是否通过调控软骨细胞自噬介导的纤毛生成来影响OA的发展。我们发现这两种分子都抑制了软骨细胞的这一过程。此外,细胞内钙和活性氧(ROS)水平同时升高。此外,我们还探讨了软骨细胞自噬与纤毛生成之间的关系。雷帕霉素对自噬的激活作用明显减轻了OLA和LA对纤毛生成的抑制作用。重要的是,下调软骨细胞中 AKT 和 mTOR 的表达可逆转自噬介导的纤毛生成抑制以及 OLA 和 LA 诱导的 ROS 积累介导的炎症。综上所述,我们的研究结果表明,在 OA 发病过程中,OLA 和 LA 通过自噬介导的纤毛生成障碍诱导钙超载驱动的 ROS 积累。这些研究结果表明,针对软骨细胞中的自噬和纤毛生成是一种保护性策略,可防止 OLA 和 LA 诱导的 OA 发病。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

VIEW
Multiple-
CiteScore
12.60
自引率
2.30%
发文量
0
审稿时长
10 weeks
期刊介绍:
View publishes scientific articles studying novel crucial contributions in the areas of Biomaterials and General Chemistry. View features original academic papers which go through peer review by experts in the given subject area.View encourages submissions from the research community where the priority will be on the originality and the practical impact of the reported research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


