Compounds with Chelating 3-Dimethylamino-1-propyl Ligands as Chemical Vapor Deposition Precursors. Synthesis and Characterization of M[(CH2)3NMe2]2 Complexes of Nickel(II), Palladium(II), and Platinum(II)
IF 2.5
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
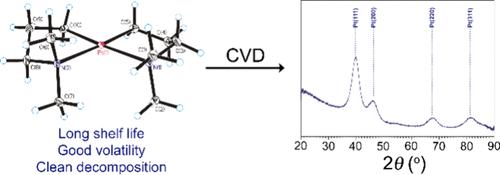
We describe the synthesis and characterization of three new square-planar compounds of stoichiometry M[(CH2)3NMe2]2, where M = Ni, Pd, or Pt, each of which contains two chelating 3-dimethylamino-1-propyl ligands. The nickel(II) and palladium(II) compounds decompose above −78 and 0 °C, respectively, but the platinum(II) compound has a thermolysis onset temperature of 130 °C. The Pd and Pt complexes are dynamic in solution: they undergo ring inversion with small free energies of activation of ΔG⧧ = 7.9 ± 0.1 and 8.3 ± 0.1 kcal mol–1, respectively, at 298 K. The Pt complex sublimes at 40 °C and 5 mTorr. In benzene solution, the Pt compound thermolyzes primarily through β-hydrogen elimination; 80 ± 10% of the hydrogen atoms and 75 ± 5% of the carbon atoms from the precursor can be accounted for in the byproducts. The thermolysis of the Pt complex in C6D6 follows first-order kinetics, with an activation free energy ΔG⧧ of 29.9 ± 0.1 kcal mol–1 at 110 °C. Under CVD conditions, thin films grown of the Pt complex at 200 °C contain nanocrystalline Pt; analysis of the film growth byproducts suggest that the main decomposition pathway involves β-hydrogen elimination and reductive elimination steps, as seen in solution.
作为化学气相沉积前驱体的 3-二甲基氨基-1-丙基螯合配体化合物。镍(II)、钯(II)和铂(II)的 M[(CH2)3NMe2]2配合物的合成与表征
我们描述了三种新的方形平面化合物的合成和特性,其化学计量学为 M[(CH2)3NMe2]2,其中 M = 镍、钯或铂,每种化合物都含有两个螯合 3-二甲氨基-1-丙基配体。镍(II)和钯(II)化合物分别在-78℃和 0℃以上分解,但铂(II)化合物的热分解起始温度为 130℃。钯和铂络合物在溶液中是动态的:它们在 298 K 时发生环反转,活化自由能分别为 ΔG⧧ = 7.9 ± 0.1 和 8.3 ± 0.1 kcal mol-1。在苯溶液中,铂化合物主要通过β-氢消除而发生热解;副产物中含有前驱体中 80 ± 10% 的氢原子和 75 ± 5% 的碳原子。铂复合物在 C6D6 中的热解遵循一阶动力学,在 110 °C 时的活化自由能 ΔG⧧ 为 29.9 ± 0.1 kcal mol-1。在化学气相沉积条件下,铂络合物在 200 °C 生长出的薄膜含有纳米铂晶体;对薄膜生长副产物的分析表明,主要分解途径包括β-氢消除和还原消除步骤,正如在溶液中看到的那样。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organometallics
化学-无机化学与核化学
CiteScore
5.60
自引率
7.10%
发文量
382
审稿时长
1.7 months
期刊介绍:
Organometallics is the flagship journal of organometallic chemistry and records progress in one of the most active fields of science, bridging organic and inorganic chemistry. The journal publishes Articles, Communications, Reviews, and Tutorials (instructional overviews) that depict research on the synthesis, structure, bonding, chemical reactivity, and reaction mechanisms for a variety of applications, including catalyst design and catalytic processes; main-group, transition-metal, and lanthanide and actinide metal chemistry; synthetic aspects of polymer science and materials science; and bioorganometallic chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


