Blockade of thromboxane A2 signaling attenuates ethanol-induced myocardial inflammatory response in mice
Abstract
Background
Alcohol-associated cardiomyopathy (ACM) is a cardiac muscle disease characterized by inflammation and oxidative stress. Thromboxane-prostanoid receptor (TP-R) plays an important role in the pathogenesis of cardiovascular disease. Herein, we hypothesize that TP-R mediates alcohol-induced early cardiac injury.
Methods
Eight-week-old male C57BL/6 wild-type mice were fed a chronic ethanol (ET) or control diet (CON) for 10 days followed by a single binge of ethanol or maltose-dextrin through oral gavage. A cohort of ethanol-fed mice received SQ 29,548 (SQ), a TP-R antagonist. RNA sequencing, real-time PCR, and western blot analysis were performed on left ventricle to investigate alterations in genes and/or proteins mediating oxidative stress, inflammation, and cardiac remodeling. Sirius Red staining was performed to measure myocardial fibrosis.
Results
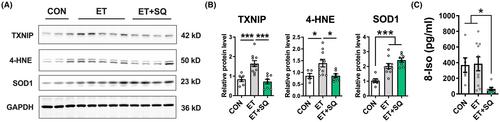
RNA-sequencing analysis of myocardium from CON and ET groups identified 142 genes that were significantly altered between the two groups. In particular, the gene expression of thioredoxin-interacting protein (TXNIP), a component of NLR family pyrin domain containing 3 (NLRP3) signaling, which mediates oxidative stress and inflammatory response, was upregulated in response to ethanol exposure. The myocardial protein levels of TP-R and thromboxane A2 synthase were increased upon alcohol exposure. Ethanol increased the levels of 4-hydroxynonenal, a marker of oxidative stress, with a concomitant increase in the protein levels of TXNIP and NLRP3, and administration of SQ attenuated these effects. Additionally, ethanol increased the protein levels of pro-inflammatory mediators, including tumor necrosis factor alpha and the NLRP3 downstream product, secretory interleukin 1 beta, and SQ blunted these effects. Finally, the Sirius red staining of the myocardium revealed an increase in collagen deposition in ethanol-fed mice which was attenuated by TP-R antagonism.
Conclusion
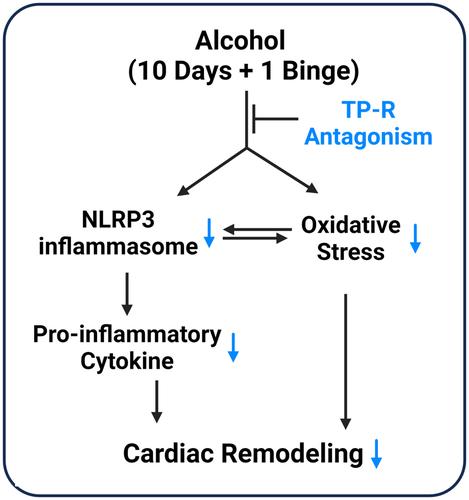
This study demonstrates that ethanol promotes the NLRP3 signaling pathway within the myocardium, leading to a pro-inflammatory milieu that potentially initiates early myocardial remodeling, and TP-R antagonism attenuates this effect.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


