RNA modification gene WDR4 facilitates tumor progression and immunotherapy resistance in breast cancer
IF 13
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
Introduction
Growing interest toward RNA modification in cancer has inspired the exploration of gene sets related to multiple RNA modifications. However, a comprehensive elucidation of the clinical value of various RNA modifications in breast cancer is still lacking.
Objectives
This study aimed to provide a strategy based on RNA modification-related genes for predicting therapy response and survival outcomes in breast cancer patients.
Methods
Genes related to thirteen RNA modification patterns were integrated for establishing a nine-gene-containing signature-RMscore. Alterations of tumor immune microenvironment and therapy response featured by different RMscore levels were assessed by bulk transcriptome, single-cell transcriptome and genomics analyses. The biological function of key RMscore-related molecules was investigated by cellular experiments in vitro and in vivo, using flow cytometry, immunohistochemistry and immunofluorescence staining.
Results
This study has raised an effective therapy strategy for breast cancer patients after a well-rounded investigation of RNA modification-related genes. With a great performance of predicting patient prognosis, high levels of the RMscore proposed in this study represented suppressive immune microenvironment and therapy resistance, including adjuvant chemotherapy and PD-L1 blockade treatment. As the key contributor of the RMscore, inhibition of WDR4 impaired breast cancer progression significantly in vitro and in vivo, as well as participated in regulating cell cycle and mTORC1 signaling pathway via m7G modification.
Conclusion
Briefly, this study has developed promising and effective tactics to achieve the prediction of survival probabilities and treatment response in breast cancer patients.

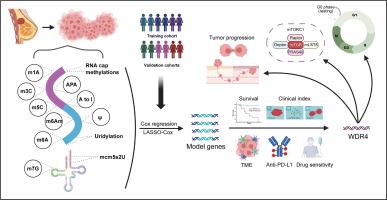
RNA 修饰基因 WDR4 有助于乳腺癌的肿瘤进展和免疫疗法抗药性。
导言:人们对癌症中的 RNA 修饰越来越感兴趣,这激发了人们对多种 RNA 修饰相关数据集的探索。然而,目前仍缺乏对乳腺癌中各种 RNA 修饰临床价值的全面阐释:本研究旨在提供一种基于 RNA 修饰相关基因的策略,用于预测乳腺癌患者的治疗反应和生存结果:方法:整合了13种RNA修饰模式的相关基因,建立了包含9个基因的特征-RMscore。通过大量转录组、单细胞转录组和基因组学分析,评估了不同RMscore水平下肿瘤免疫微环境的改变和治疗反应。利用流式细胞术、免疫组织化学和免疫荧光染色等方法,通过体外和体内细胞实验研究了RMscore相关关键分子的生物学功能:本研究通过对 RNA 修饰相关基因的全面研究,为乳腺癌患者提出了一种有效的治疗策略。本研究中提出的高水平 RMscore 代表了抑制性免疫微环境和治疗耐药,包括辅助化疗和 PD-L1 阻断治疗。作为RMscore的主要贡献者,抑制WDR4会显著影响乳腺癌在体外和体内的进展,并通过m7G修饰参与调控细胞周期和mTORC1信号通路:简而言之,这项研究为预测乳腺癌患者的生存概率和治疗反应提供了有效的方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Advanced Research
Multidisciplinary-Multidisciplinary
CiteScore
21.60
自引率
0.90%
发文量
280
审稿时长
12 weeks
期刊介绍:
Journal of Advanced Research (J. Adv. Res.) is an applied/natural sciences, peer-reviewed journal that focuses on interdisciplinary research. The journal aims to contribute to applied research and knowledge worldwide through the publication of original and high-quality research articles in the fields of Medicine, Pharmaceutical Sciences, Dentistry, Physical Therapy, Veterinary Medicine, and Basic and Biological Sciences.
The following abstracting and indexing services cover the Journal of Advanced Research: PubMed/Medline, Essential Science Indicators, Web of Science, Scopus, PubMed Central, PubMed, Science Citation Index Expanded, Directory of Open Access Journals (DOAJ), and INSPEC.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


