Macrophage exosomes mediate palmitic acid-induced metainflammation by transferring miR-3064-5p to target IκBα and activate NF-κB signaling
IF 11.4
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
Introduction
High palmitic acid (PA) levels trigger metainflammation, facilitating the onset and progression of chronic metabolic diseases. Recently, exosomes were identified as new inflammation mediators. However, the mechanism by which macrophage exosomes mediate PA-induced inflammation remains unclear.
Objectives
To explore how PA induces metainflammation through macrophage exosomes.
Methods
Exosomes secreted by RAW264.7 mouse macrophages stimulated with PA (ExosPA) or not (Exos) were prepared by ultracentrifugation. The differential miRNAs between ExosPA and Exos were identified by high-throughput sequencing, and their targeted mRNAs and proteins were bioinformatically analyzed and verified by qPCR and western blot. Mouse macrophages and metabolic cells (AML-12 hepatocytes, C2C12 myocytes or 3T3-L1 adipocytes) were treated with ExosPA or Exos. The verified miRNAs and its targeted molecules related to inflammation were analyzed in recipient cells. Furthers, exosomes were prepared from primary peritoneal macrophages isolated from AIN93G diet-fed (Control PM-Exos) or HPD-fed (PA PM-Exos) mice. Control or PA PM-Exos were then tail vein injected (30 μg) into mice (n = 10), once a week for 2 weeks. The verified miRNA and its targets in blood, blood exosomes, and metabolic tissues were detected. Finally, measured the levels of miRNA, inflammatory factors, and fatty acids in the blood of 20 obese/overweight individuals and 20 healthy individuals.
Results
ExoPA activate NF-κB signaling and enhance inflammatory enzyme/cytokine production in macrophages and metabolic cells. ExoPA enrich miR-3064-5p and target to inhibit IκBα as verified by exosome inhibitors and miR-3064-5p mimics and inhibitors. HPD elevates exosomal miR-3064-5p, macrophage exosomal miR-3064-5p, and inflammatory cytokine levels in mice circulation. PA PM-Exos from HPD-fed mice triggered inflammation in the circulation and metabolic tissues/organs of chow diet-fed mice. Overweight/obese individuals exhibit increased levels of circulating palmitoleic acid, exosomal miR-3064-5p, and high-sensitivity C-reactive proteins.
Conclusions
Macrophage exosomes transferring miR-3064-5p to target IκBα and activate NF-κB signaling in metabolic cells is a mechanism of PA-induced metainflammation.
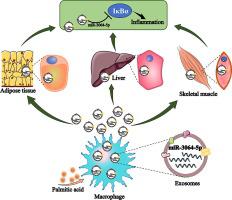

巨噬细胞外泌体通过转移 miR-3064-5p 靶向 IκBα 和激活 NF-κB 信号,介导棕榈酸诱导的变态反应性炎症。
导言:高棕榈酸(PA)水平会引发变态反应性炎症,促进慢性代谢性疾病的发生和发展。最近,外泌体被确认为新的炎症介质。然而,巨噬细胞外泌体介导 PA 诱导炎症的机制仍不清楚:探讨 PA 如何通过巨噬细胞外泌体诱导变态反应性炎症:方法:采用超速离心法制备受 PA 刺激或未受 PA 刺激的 RAW264.7 小鼠巨噬细胞分泌的外泌体(ExosPA)。通过高通量测序鉴定了 ExosPA 和 Exos 的不同 miRNAs,对其靶标 mRNAs 和蛋白质进行了生物信息学分析,并通过 qPCR 和 Western 印迹进行了验证。用 ExosPA 或 Exos 处理小鼠巨噬细胞和代谢细胞(AML-12 肝细胞、C2C12 肌细胞或 3T3-L1 脂肪细胞)。在受体细胞中分析了已验证的 miRNAs 及其与炎症相关的靶分子。此外,还从 AIN93G 膳食喂养(Control PM-Exos)或 HPD 喂养(PA PM-Exos)小鼠分离的初级腹腔巨噬细胞中制备了外泌体。然后将对照组或 PA PM-Exos 小鼠(n = 10)尾静脉注射(30 μg),每周一次,连续 2 周。检测血液、血液外泌体和代谢组织中已验证的 miRNA 及其靶标。最后,测量了 20 名肥胖/超重者和 20 名健康者血液中的 miRNA、炎症因子和脂肪酸水平:结果:ExoPA能激活巨噬细胞和代谢细胞中的NF-κB信号,并增强炎症酶/细胞因子的产生。通过外泌体抑制剂和 miR-3064-5p 模拟物和抑制剂证实,外泌体PA 富集 miR-3064-5p,并以抑制 IκBα 为目标。HPD 可提高小鼠循环中的外泌体 miR-3064-5p、巨噬细胞外泌体 miR-3064-5p 和炎性细胞因子水平。HPD喂养的小鼠的PA PM-外泌体引发了饲料喂养的小鼠血液循环和代谢组织/器官中的炎症。超重/肥胖者的循环中棕榈油酸、外泌体miR-3064-5p和高敏C反应蛋白水平都会升高:结论:巨噬细胞外泌体转移 miR-3064-5p 靶向 IκBα 并激活代谢细胞中的 NF-κB 信号,是 PA 诱导代谢炎的一种机制。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Advanced Research
Multidisciplinary-Multidisciplinary
CiteScore
21.60
自引率
0.90%
发文量
280
审稿时长
12 weeks
期刊介绍:
Journal of Advanced Research (J. Adv. Res.) is an applied/natural sciences, peer-reviewed journal that focuses on interdisciplinary research. The journal aims to contribute to applied research and knowledge worldwide through the publication of original and high-quality research articles in the fields of Medicine, Pharmaceutical Sciences, Dentistry, Physical Therapy, Veterinary Medicine, and Basic and Biological Sciences.
The following abstracting and indexing services cover the Journal of Advanced Research: PubMed/Medline, Essential Science Indicators, Web of Science, Scopus, PubMed Central, PubMed, Science Citation Index Expanded, Directory of Open Access Journals (DOAJ), and INSPEC.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


