Inhibition of M. tuberculosis and human ATP synthase by BDQ and TBAJ-587
IF 48.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
Bedaquiline (BDQ), a first-in-class diarylquinoline anti-tuberculosis drug, and its analogue, TBAJ-587, prevent the growth and proliferation of Mycobacterium tuberculosis by inhibiting ATP synthase1,2. However, BDQ also inhibits human ATP synthase3. At present, how these compounds interact with either M. tuberculosis ATP synthase or human ATP synthase is unclear. Here we present cryogenic electron microscopy structures of M. tuberculosis ATP synthase with and without BDQ and TBAJ-587 bound, and human ATP synthase bound to BDQ. The two inhibitors interact with subunit a and the c-ring at the leading site, c-only sites and lagging site in M. tuberculosis ATP synthase, showing that BDQ and TBAJ-587 have similar modes of action. The quinolinyl and dimethylamino units of the compounds make extensive contacts with the protein. The structure of human ATP synthase in complex with BDQ reveals that the BDQ-binding site is similar to that observed for the leading site in M. tuberculosis ATP synthase, and that the quinolinyl unit also interacts extensively with the human enzyme. This study will improve researchers’ understanding of the similarities and differences between human ATP synthase and M. tuberculosis ATP synthase in terms of the mode of BDQ binding, and will allow the rational design of novel diarylquinolines as anti-tuberculosis drugs. Cryogenic electron microscopy structures of Mycobacterium tuberculosis ATP synthase and human ATP synthase bound to the anti-tuberculosis drug bedaquiline or its analogue TBAJ-587 shed light on drug binding and could lead to new treatments for tuberculosis.
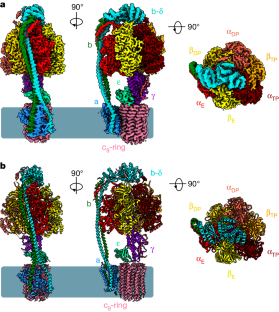
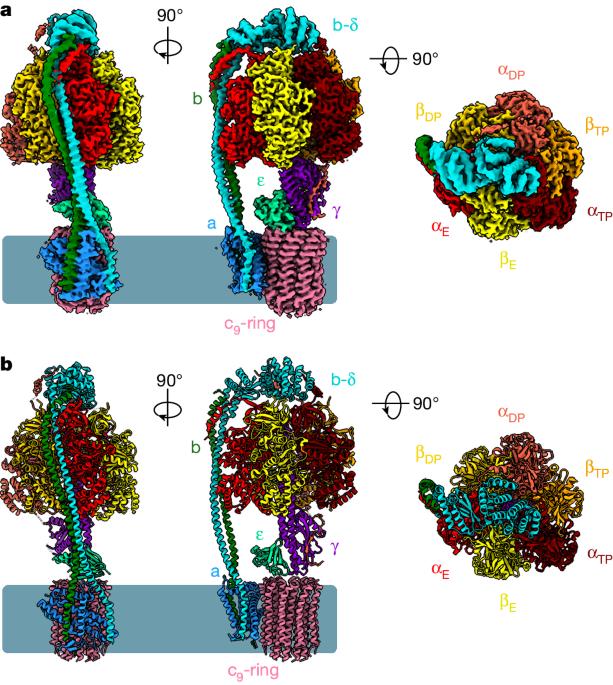
BDQ 和 TBAJ-587 对结核杆菌和人类 ATP 合成酶的抑制作用。
贝达喹啉(BDQ)是第一类二芳基喹啉类抗结核药物,其类似物 TBAJ-587 通过抑制 ATP 合成酶阻止结核分枝杆菌的生长和增殖1,2。不过,BDQ 也能抑制人类 ATP 合成酶3。目前,这些化合物如何与结核杆菌 ATP 合成酶或人类 ATP 合成酶相互作用尚不清楚。在此,我们展示了与 BDQ 和 TBAJ-587 结合或未结合的结核杆菌 ATP 合成酶以及与 BDQ 结合的人类 ATP 合成酶的低温电子显微镜结构。这两种抑制剂在结核杆菌 ATP 合成酶的前导位点、仅 c 位点和滞后位点与 a 亚基和 c 环相互作用,表明 BDQ 和 TBAJ-587 具有相似的作用模式。化合物的喹啉基和二甲基氨基单元与蛋白质有广泛的接触。人类 ATP 合成酶与 BDQ 复合物的结构显示,BDQ 结合位点与在结核杆菌 ATP 合成酶中观察到的主导位点相似,而且喹啉基单元也与人类酶发生了广泛的相互作用。这项研究将加深研究人员对人类 ATP 合成酶与 M. tuberculosis ATP 合成酶在 BDQ 结合模式方面异同的了解,并有助于合理设计新型二芳基喹啉类抗结核药物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature
综合性期刊-综合性期刊
CiteScore
90.00
自引率
1.20%
发文量
3652
审稿时长
3 months
期刊介绍:
Nature is a prestigious international journal that publishes peer-reviewed research in various scientific and technological fields. The selection of articles is based on criteria such as originality, importance, interdisciplinary relevance, timeliness, accessibility, elegance, and surprising conclusions. In addition to showcasing significant scientific advances, Nature delivers rapid, authoritative, insightful news, and interpretation of current and upcoming trends impacting science, scientists, and the broader public. The journal serves a dual purpose: firstly, to promptly share noteworthy scientific advances and foster discussions among scientists, and secondly, to ensure the swift dissemination of scientific results globally, emphasizing their significance for knowledge, culture, and daily life.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


