The dichapetalins and dichapetalin-type compounds: structural diversity, bioactivity, and future research perspectives†
IF 10.2
1区 化学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Covering mainly from 2013 up to 2023 with relevant references to work done before 2013
First reported in 1995, the dichapetalins and analogous compounds constitute a novel class of natural dammarane-type merotriterpenoids characterized by their unique 2-phenylpyrano moiety annellated to ring A of the dammarane skeleton. They have been reported from only two genera: Dichapetalum (Dichapetalaceae) and Phyllanthus (Phyllanthaceae). About 100 novel dichapetalins and dichapetalin-type compounds, including the acutissimatriterpenes and their antitumour and other bioactivities have been reported. In the present review, we cover the distribution, ethnobotanical and medicinal importance and the diversity of secondary metabolites reported from the two genera Dichapetalum and Phyllanthus from 2013 to date, with appropriate reference to relevant information prior to 2013. We also propose and discuss possible biosynthetic pathways, antitumour activity against a broad range of human and murine cancer cell lines, structure activity relationships, and other biological activities and mechanisms of action. Finally, the review deals with future perspectives which include expansion of the structural diversity and bioactivity scope, possible simplification of the structural complexity of the compounds to enhance their drug-likeness, in silico studies, and continuation of the search for new dichapetalins and dichapetalin-type compounds from the over 200 Dichapetalum and over 1200 Phyllanthus species yet to be investigated. It is envisaged that the present review will stimulate further multidisciplinary and interdisciplinary studies.
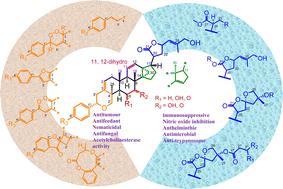
二氢杨梅素和二氢杨梅素类化合物:结构多样性、生物活性和未来研究前景。
主要涵盖 2013 年至 2023 年的内容,并附有 2013 年之前所做工作的相关参考文献。1995 年首次报道的二廿八烷和类似化合物构成了一类新型的天然达玛烷型三萜类化合物,其特点是在达玛烷骨架的 A 环上具有独特的 2-苯基吡喃分子。目前仅有两个属报道过这种物质:Dichapetalum 属(Dichapetalaceae)和 Phyllanthus 属(Phyllanthusaceae)。目前已报道了约 100 种新型的二柴胡苷和二柴胡苷类化合物,其中包括金合欢苷及其抗肿瘤和其他生物活性。在本综述中,我们介绍了 2013 年至今从 Dichapetalum 和 Phyllanthus 这两个属中报道的次生代谢物的分布、民族植物学和药用重要性以及多样性,并适当参考了 2013 年之前的相关信息。我们还提出并讨论了可能的生物合成途径、对多种人类和鼠类癌症细胞系的抗肿瘤活性、结构活性关系以及其他生物活性和作用机制。最后,本综述探讨了未来的发展前景,包括扩大结构多样性和生物活性的范围,简化化合物结构的复杂性以提高其药物亲和性,进行硅学研究,以及继续从尚未研究的 200 多种 Dichapetalum 和 1200 多种 Phyllanthus 品种中寻找新的 dichapetalins 和 dichapetalin 类化合物。预计本综述将促进进一步的多学科和跨学科研究。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Natural Product Reports
化学-生化与分子生物学
CiteScore
21.20
自引率
3.40%
发文量
127
审稿时长
1.7 months
期刊介绍:
Natural Product Reports (NPR) serves as a pivotal critical review journal propelling advancements in all facets of natural products research, encompassing isolation, structural and stereochemical determination, biosynthesis, biological activity, and synthesis.
With a broad scope, NPR extends its influence into the wider bioinorganic, bioorganic, and chemical biology communities. Covering areas such as enzymology, nucleic acids, genetics, chemical ecology, carbohydrates, primary and secondary metabolism, and analytical techniques, the journal provides insightful articles focusing on key developments shaping the field, rather than offering exhaustive overviews of all results.
NPR encourages authors to infuse their perspectives on developments, trends, and future directions, fostering a dynamic exchange of ideas within the natural products research community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


