Investigating druggable kinases for targeted therapy in retinoblastoma
IF 2.5
3区 生物学
Q2 GENETICS & HEREDITY
引用次数: 0
Abstract
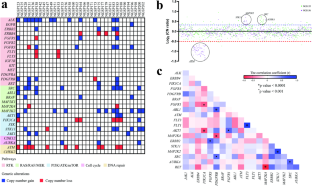
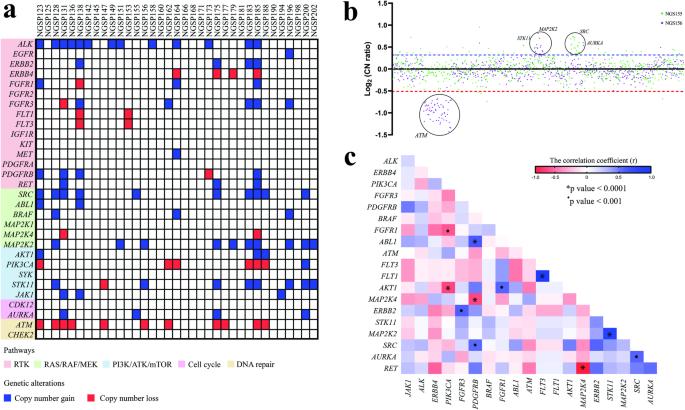
Retinoblastoma (RB) is a childhood retinal neoplasm and commonly treated with cytotoxic chemotherapeutic agents. However, these therapeutic approaches often lead to diverse adverse effects. A precise molecular therapy will alleviate these side effects and offer better treatment outcomes. Over the years, kinases have become potential drug targets in cancer therapy. Hence, we aimed to investigate genetic alterations of putative kinase drug targets in RB. Targeted exome sequencing was performed on 35 RB tumors with paired blood samples using a gene panel consisting of 29 FDA-approved kinase genes. Single nucleotide variants were analyzed for pathogenicity using an in-house pipeline and copy number variations (CNVs) were detected by a depth of coverage and CNVPanelizer. The correlation between genetic changes and clinicopathological features was assessed using GraphPad Prism. Three somatic mutations, two in ERBB4 and one in EGFR were identified. Two of these mutations (ERBB4 c.C3836A & EGFR c.A1196T) were not reported earlier. CNV analysis revealed recurrent gains of ALK, MAP2K2, SRC, STK11, and FGFR3 as well as frequent losses of ATM, PI3KCA and ERBB4. Notably, nonresponsive tumors had a higher incidence of amplifications in clinically actionable genes such as ALK. Moreover, ALK gain and ATM loss were strongly correlated with optic nerve head invasion. In conclusion, our study revealed genetic alterations of druggable kinases in RB, providing preliminary insights for the exploration of kinase-targeted therapy in RB.
研究可用于视网膜母细胞瘤靶向治疗的药物激酶。
视网膜母细胞瘤(RB)是一种儿童视网膜肿瘤,通常采用细胞毒性化疗药物进行治疗。然而,这些治疗方法往往会导致各种不良反应。精确的分子疗法将减轻这些副作用,并提供更好的治疗效果。多年来,激酶已成为癌症治疗的潜在药物靶点。因此,我们旨在研究 RB 中潜在激酶药物靶点的基因改变。我们对 35 例 RB 肿瘤和配对的血液样本进行了靶向外显子组测序,使用了由 29 个 FDA 批准的激酶基因组成的基因面板。利用内部流水线分析了单核苷酸变异的致病性,并通过覆盖深度和CNVPanelizer检测了拷贝数变异(CNV)。使用 GraphPad Prism 评估了基因变化与临床病理特征之间的相关性。发现了三个体细胞突变,其中两个在ERBB4中,一个在表皮生长因子受体中。其中两个突变(ERBB4 c.C3836A和表皮生长因子受体c.A1196T)以前没有报道过。CNV 分析显示,ALK、MAP2K2、SRC、STK11 和 FGFR3 基因反复增殖,ATM、PI3KCA 和 ERBB4 基因频繁丢失。值得注意的是,非反应性肿瘤中ALK等临床可操作基因的扩增发生率较高。此外,ALK增益和ATM缺失与视神经头侵犯密切相关。总之,我们的研究揭示了RB中可药用激酶的基因改变,为探索RB的激酶靶向治疗提供了初步见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Human Genetics
生物-遗传学
CiteScore
7.20
自引率
0.00%
发文量
101
审稿时长
4-8 weeks
期刊介绍:
The Journal of Human Genetics is an international journal publishing articles on human genetics, including medical genetics and human genome analysis. It covers all aspects of human genetics, including molecular genetics, clinical genetics, behavioral genetics, immunogenetics, pharmacogenomics, population genetics, functional genomics, epigenetics, genetic counseling and gene therapy.
Articles on the following areas are especially welcome: genetic factors of monogenic and complex disorders, genome-wide association studies, genetic epidemiology, cancer genetics, personal genomics, genotype-phenotype relationships and genome diversity.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


