Leptin-mediated suppression of lipoprotein lipase cleavage enhances lipid uptake and facilitates lymph node metastasis in gastric cancer
Abstract
Background
Lymph node metastasis (LNM) is the primary mode of metastasis in gastric cancer (GC). However, the precise mechanisms underlying this process remain elusive. Tumor cells necessitate lipid metabolic reprogramming to facilitate metastasis, yet the role of lipoprotein lipase (LPL), a pivotal enzyme involved in exogenous lipid uptake, remains uncertain in tumor metastasis. Therefore, the aim of this study was to investigate the presence of lipid metabolic reprogramming during LNM of GC as well as the role of LPL in this process.
Methods
Intracellular lipid levels were quantified using oil red O staining, BODIPY 493/503 staining, and flow cytometry. Lipidomics analysis was employed to identify alterations in intracellular lipid composition following LPL knockdown. Protein expression levels were assessed through immunohistochemistry, Western blotting, and enzyme-linked immunosorbent assays. The mouse popliteal LNM model was utilized to investigate differences in LNM. Immunoprecipitation and mass spectrometry were employed to examine protein associations. In vitro phosphorylation assays and Phos-tag sodium dodecyl-sulfate polyacrylamide gel electrophoresis assays were conducted to detect angiopoietin-like protein 4 (ANGPTL4) phosphorylation.
Results
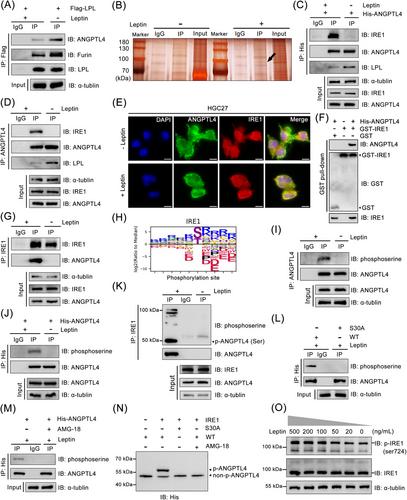
We identified that an elevated intracellular lipid level represents a crucial characteristic of node-positive (N+) GC and further demonstrated that a high-fat diet can expedite LNM. LPL was found to be significantly overexpressed in N+ GC tissues and shown to facilitate LNM by mediating dietary lipid uptake within GC cells. Leptin, an obesity-related hormone, intercepted the effect exerted by ANGPTL4/Furin on LPL cleavage. Circulating leptin binding to the leptin receptor could induce the activation of inositol-requiring enzyme-1 (IRE1) kinase, leading to the phosphorylation of ANGPTL4 at the serine 30 residue and subsequently reducing its binding affinity with LPL. Moreover, our research revealed that LPL disrupted lipid homeostasis by elevating intracellular levels of arachidonic acid, which then triggered the cyclooxygenase-2/prostaglandin E2 (PGE2) pathway, thereby promoting tumor lymphangiogenesis.
Conclusions
Leptin-induced phosphorylation of ANGPTL4 facilitates LPL-mediated lipid uptake and consequently stimulates the production of PGE2, ultimately facilitating LNM in GC.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


