Direct neuronal reprogramming of mouse astrocytes is associated with multiscale epigenome remodeling and requires Yy1
IF 21.2
1区 医学
Q1 NEUROSCIENCES
引用次数: 0
Abstract
Direct neuronal reprogramming is a promising approach to regenerate neurons from local glial cells. However, mechanisms of epigenome remodeling and co-factors facilitating this process are unclear. In this study, we combined single-cell multiomics with genome-wide profiling of three-dimensional nuclear architecture and DNA methylation in mouse astrocyte-to-neuron reprogramming mediated by Neurogenin2 (Ngn2) and its phosphorylation-resistant form (PmutNgn2), respectively. We show that Ngn2 drives multilayered chromatin remodeling at dynamic enhancer–gene interaction sites. PmutNgn2 leads to higher reprogramming efficiency and enhances epigenetic remodeling associated with neuronal maturation. However, the differences in binding sites or downstream gene activation cannot fully explain this effect. Instead, we identified Yy1, a transcriptional co-factor recruited by direct interaction with Ngn2 to its target sites. Upon deletion of Yy1, activation of neuronal enhancers, genes and ultimately reprogramming are impaired without affecting Ngn2 binding. Thus, our work highlights the key role of interactors of proneural factors in direct neuronal reprogramming. The molecular mechanisms underlying direct neuronal reprogramming are unclear. Here the authors show Ngn2-mediated chromatin remodeling and its binding sites underlying mouse astrocyte-to-neuron reprogramming and identify Yy1, a transcription co-factor, as an important regulator.
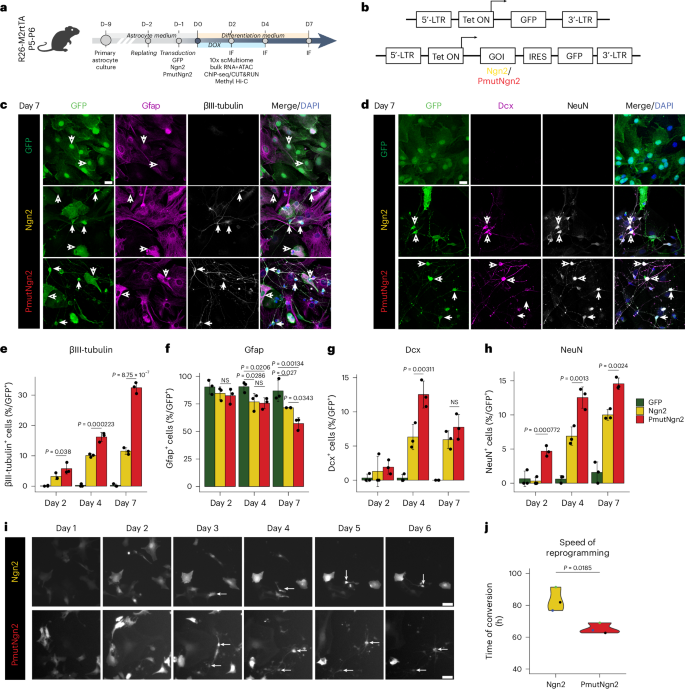
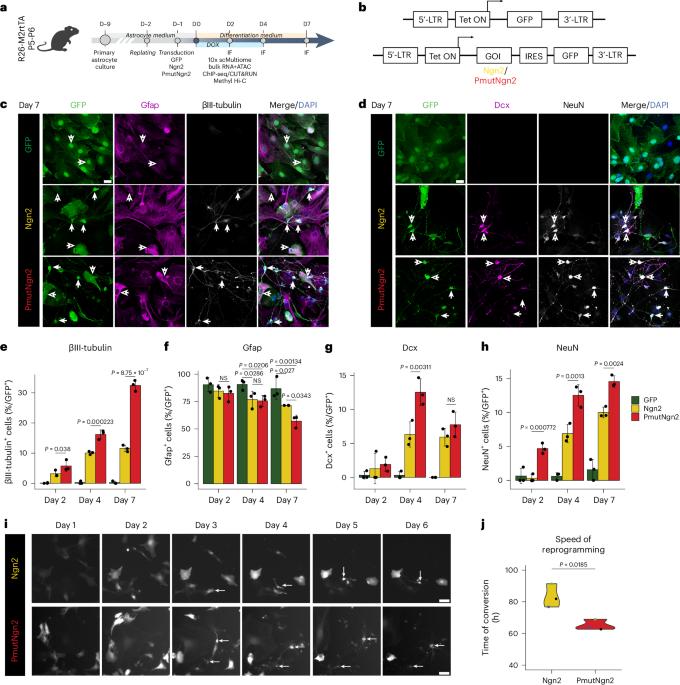
小鼠星形胶质细胞的直接神经元重编程与多尺度表观基因组重塑有关,需要 Yy1
直接进行神经元重编程是一种从局部胶质细胞再生神经元的可行方法。然而,表观基因组重塑的机制和促进这一过程的辅助因子尚不清楚。在这项研究中,我们将单细胞多组学与小鼠星形胶质细胞到神经元重编程过程中分别由神经原蛋白2(Ngn2)及其磷酸化抗性形式(PmutNgn2)介导的三维核结构和DNA甲基化的全基因组谱分析结合起来。我们发现,Ngn2 在动态增强子-基因相互作用位点上驱动多层染色质重塑。PmutNgn2导致更高的重编程效率,并增强了与神经元成熟相关的表观遗传重塑。然而,结合位点或下游基因激活的差异并不能完全解释这种效应。相反,我们发现了一种转录辅助因子 Yy1,它通过与 Ngn2 的直接相互作用被招募到其靶位点。删除 Yy1 后,神经元增强子、基因的激活以及最终的重编程都会受到影响,而不会影响 Ngn2 的结合。因此,我们的工作凸显了朊病毒因子的相互作用者在直接神经元重编程中的关键作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature neuroscience
医学-神经科学
CiteScore
38.60
自引率
1.20%
发文量
212
审稿时长
1 months
期刊介绍:
Nature Neuroscience, a multidisciplinary journal, publishes papers of the utmost quality and significance across all realms of neuroscience. The editors welcome contributions spanning molecular, cellular, systems, and cognitive neuroscience, along with psychophysics, computational modeling, and nervous system disorders. While no area is off-limits, studies offering fundamental insights into nervous system function receive priority.
The journal offers high visibility to both readers and authors, fostering interdisciplinary communication and accessibility to a broad audience. It maintains high standards of copy editing and production, rigorous peer review, rapid publication, and operates independently from academic societies and other vested interests.
In addition to primary research, Nature Neuroscience features news and views, reviews, editorials, commentaries, perspectives, book reviews, and correspondence, aiming to serve as the voice of the global neuroscience community.
文献相关原料
| 公司名称 | 产品信息 | 采购帮参考价格 |
|---|
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


