Sequence-specific interactions determine viscoelasticity and ageing dynamics of protein condensates
IF 17.6
1区 物理与天体物理
Q1 PHYSICS, MULTIDISCIPLINARY
引用次数: 0
Abstract
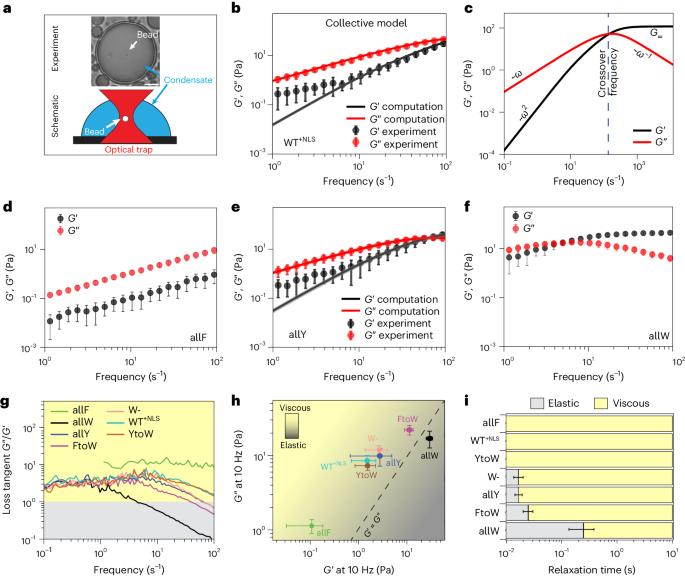
Biomolecular condensates are viscoelastic materials. Here we investigate the determinants of the sequence-encoded and age-dependent viscoelasticity of condensates formed by the prion-like low-complexity domain of the protein hnRNP A1 and its designed variants. We find that the dominantly viscous forms of the condensates are metastable Maxwell fluids. A Rouse–Zimm model that accounts for the network-like organization of proteins within condensates reproduces the measured viscoelastic moduli. We show that the strengths of aromatic inter-sticker interactions determine sequence-specific amplitudes of elastic and viscous moduli and the timescales over which elastic properties dominate. These condensates undergo physical ageing on sequence-specific timescales. This is driven by mutations to spacer residues that weaken the metastability of dominantly viscous phases. The ageing of condensates is accompanied by disorder-to-order transitions, leading to the formation of non-fibrillar, β-sheet-containing, semi-crystalline, elastic, Kelvin–Voigt solids. Our results suggest that sequence grammars, which refer to amino acid identities of stickers versus spacers in prion-like low-complexity domains, have evolved to afford control over metastabilities of dominantly viscous fluid phases of condensates. This selection is likely to render barriers for conversion from metastable fluids to globally stable solids insurmountable on functionally relevant timescales. The time-dependent viscoelastic moduli of biomolecular condensates are connected to the functions that the condensates influence in cells. Now sticker and spacer residues in proteins are shown to regulate condensate viscoelasticity and ageing dynamics.
序列特异性相互作用决定蛋白质凝聚物的粘弹性和老化动力学
生物分子凝聚物是一种粘弹性材料。在这里,我们研究了由蛋白质 hnRNP A1 的朊病毒样低复杂性结构域及其设计变体形成的凝聚体的序列编码和年龄依赖性粘弹性的决定因素。我们发现,这些凝聚物的主要粘性形式是可蜕变的麦克斯韦流体。Rouse-Zimm 模型解释了凝聚体中蛋白质的网络状组织,再现了测量到的粘弹性模量。我们的研究表明,芳香族贴纸间相互作用的强度决定了特定序列的弹性和粘性模量振幅,以及弹性特性占主导地位的时间尺度。这些凝聚物在特定序列的时间尺度上经历物理老化。这是由间隔残基的突变驱动的,突变削弱了粘性相的转移性。凝结物的老化伴随着无序到有序的转变,从而形成非纤维状、含β片状、半晶体、弹性、开尔文-伏依格特固体。我们的研究结果表明,序列语法(指朊病毒样低复杂性结构域中粘连物与间隔物的氨基酸相同性)的进化能够控制冷凝物主要粘性流体相的蜕变。这种选择可能会使从可转移流体转化为全球稳定固体的障碍在功能相关的时间尺度上变得不可逾越。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Physics
物理-物理:综合
CiteScore
30.40
自引率
2.00%
发文量
349
审稿时长
4-8 weeks
期刊介绍:
Nature Physics is dedicated to publishing top-tier original research in physics with a fair and rigorous review process. It provides high visibility and access to a broad readership, maintaining high standards in copy editing and production, ensuring rapid publication, and maintaining independence from academic societies and other vested interests.
The journal presents two main research paper formats: Letters and Articles. Alongside primary research, Nature Physics serves as a central source for valuable information within the physics community through Review Articles, News & Views, Research Highlights covering crucial developments across the physics literature, Commentaries, Book Reviews, and Correspondence.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


