Why the Mostafa Maged maneuver is better: Mostafa Maged maneuver compared with bimanual uterine compression for postpartum hemorrhage
Abstract
Background
This study aimed to investigate the effectiveness of the Mostafa Maged maneuver compared with bimanual uterine compression for the management of postpartum hemorrhage during vaginal delivery.
Methods
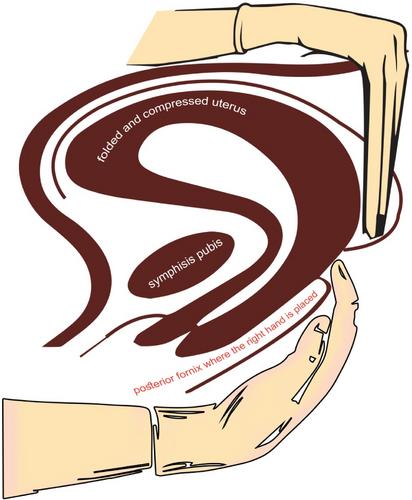
This was a prospective study involving 200 patients divided into two groups with 100 patients in each group. Group I received (Mostafa Maged maneuver), and Group II received (routine bimanual uterine compression). The Mostafa Maged maneuver requires placing the right hand in the posterior fornix and positioning the left hand on the uterine fundus and posterior uterine wall via the abdomen exteriorly. The subsequent step involves holding the entire uterus against the symphysis pubis. We determined the duration of each maneuver until practitioner fatigue and evaluated whether oxytocin was administered after compression for each maneuver.
Results
No patients required blood transfusions in Group I (Mostafa Maged maneuver), unlike in Group II, in which 17/100 patients received blood transfusions after bimanual compression. There was a statistically significant difference (p < 0.001) between the groups regarding the administration of oxytocin. The mean duration of applying the Mostafa Maged maneuver was 12.7 min (range: 11–15 min). The mean duration of bimanual uterine compression (Group II) was 4.04 min (range: 3.5–4.5 min). There was a statistically significantly shorter duration for the Mostafa Maged maneuver and shorter time to onset of fatigue compared with bimanual uterine compression (p = 0.02, and p = 0.001, respectively). In contrast, there were no statistically significant differences between both groups for the dealing with the patient and the blood transfusion rate.
Conclusion
The Mostafa Maged maneuver is easy to learn and feasible to perform compared with bimanual uterine compression. The Mostafa Maged maneuver may be a valuable addition to current conservative treatments for atonic postpartum hemorrhage, especially for obstetricians who lack the training and expertise to perform more complex procedures and those working in minimally equipped facilities.
Trial registration
Clinical trial registration, including the date of registration: NCT06002256.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


