Lignin-derived porous carbons for efficient CO2 adsorption
Abstract
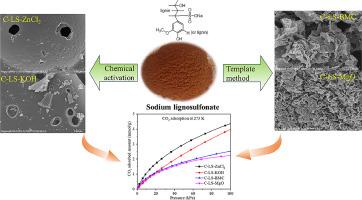
Highly efficient and stable adsorbents play a crucial role in the adsorption treatment for CO2 capture. Lignin-derived porous carbons have gained increasing attention for CO2 adsorption application due to their abundant and cost-effective raw material sources. This study involves the synthesis of a series of porous carbons derived from sodium lignosulphonate using chemical activation and template methods. It was found that synthesis methods had significant influence on the properties of the obtained porous carbons, including texture structures and surface functional groups. The pore structure of the carbons obtained through the activation methods primarily consisted of etched channels, while that of the carbons acquired via the template methods predominantly originated from stacked sheet carbon. In comparison to the carbon samples obtained from template methods, chemically-activated porous carbons exhibited higher specific surface areas (1125 m2/g for the ZnCl2-activated sample and 1998.4 m2/g for the KOH-activated sample) and more micropores. The variations in the characteristics of the carbon samples derived from lignin with different synthesis methods also affected their CO2 adsorption performance. The CO2 adsorption capability of the chemically-activated porous carbons indicated their superior suitability for CO2 adsorption due to their higher specific surface area and abundant sulfur- and oxygen-containing functional groups. Notably, the porous carbons prepared with ZnCl2 as the activation agent (C-LS-ZnCl2) exhibited the most remarkable adsorption capacity of 4.45 mmol/g at 273 K and 100 kPa, and high CO2/N2 selectivity. The adsorption-desorption cycles confirmed the remarkable stability and regeneration ability of C-LS-ZnCl2. This study findings suggest that the utilization of the ZnCl2 activation method exhibits significant potential in transforming industrial lignin waste (sodium lignosulphonate) into a suitable adsorbent for CO2 adsorption.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


