Synthesis and self-assembly of high grafting density core-shell bottlebrush polymer with amphiphilic side chain
Abstract
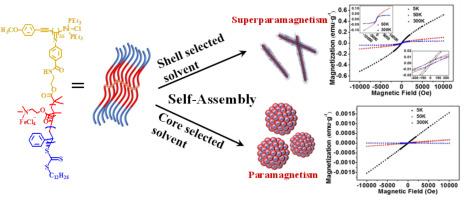
Self-assembly of bottlebrush polymers are widely studied in photonic bandgap materials, biomedical materials and nanomaterial with different structures. In this work, high grafting density bottlebrush polymer with amphiphilic QPDMA[FeCl4]-b-PS side chains grafting on every carbon atom of the backbone are prepared via grafting from approach. The para-isocyanobenzoate monomer modified with chain transfer agent (CTA) is designed and synthesized to prepared polymer backbones with CTA grafting on each atoms. The core-shell bottlebrush block copolymer is prepared by sequential reversible addition-fragmentation chain transfer polymerization of dimethylaminoethyl methacrylate and styrene. The inner core follows quaternization and ion exchange to form water soluble QPDMA[FeCl4] magnetic block. The resulting core-shell bottlebrush magnetic polymer (PCN-g-[QPDMA[FeCl4]-b-PS]) self-assembles into nanowires with magnetic QPDMA[FeCl4] as core and PS as corona in the shell selected solvent dichloromethane, the length of nanowires increases with self-assembly time. While it self-assembles into nanoparticle clusters in core selected aqueous solution. And, the thermal and magnetic properties are affected by the self-assembly morphology. Especially, the as prepared PCN-g-[QPDMA[FeCl4]-b-PS] and the nanoparticles cluster self-assembly are paramagnetic, but the nanowire self-assembly is superparamagnetic.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


