YAP1 inhibits the senescence of alveolar epithelial cells by targeting Prdx3 to alleviate pulmonary fibrosis
IF 9.5
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
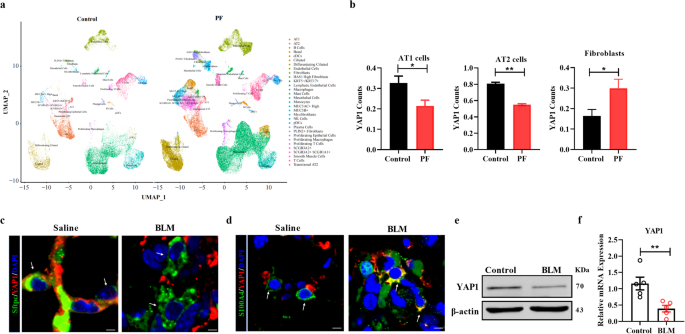
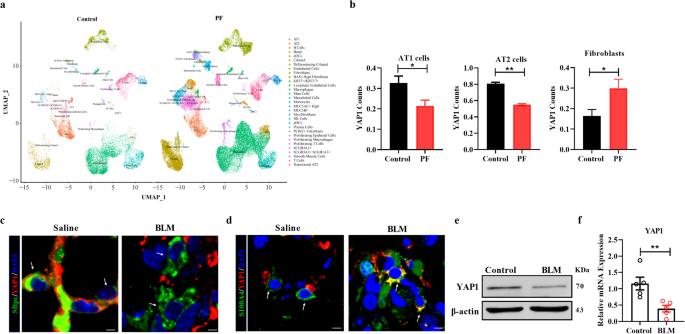
The senescence of alveolar type II (AT2) cells impedes self-repair of the lung epithelium and contributes to lung injury in the setting of idiopathic pulmonary fibrosis (IPF). Yes-associated protein 1 (YAP1) is essential for cell growth and organ development; however, the role of YAP1 in AT2 cells during pulmonary fibrosis is still unclear. YAP1 expression was found to be downregulated in the AT2 cells of PF patients. Deletion of YAP1 in AT2 cells resulted in lung injury, exacerbated extracellular matrix (ECM) deposition, and worsened lung function. In contrast, overexpression of YAP1 in AT2 cells promoted alveolar regeneration, mitigated pulmonary fibrosis, and improved lung function. In addition, overexpression of YAP1 alleviated bleomycin (BLM) -induced senescence of alveolar epithelial cells both in vivo and in vitro. Moreover, YAP1 promoted the expression of peroxiredoxin 3 (Prdx3) by directly interacting with TEAD1. Forced expression of Prdx3 inhibited senescence and improved mitochondrial dysfunction in BLM-treated MLE-12 cells, whereas depletion of Prdx3 partially abrogated the protective effect of YAP1. Furthermore, overexpression of Prdx3 facilitated self-repair of the injured lung and reduced ECM deposition, while silencing Prdx3 attenuated the antifibrotic effect of YAP1. In conclusion, this study demonstrated that YAP1 alleviates lung injury and pulmonary fibrosis by regulating Prdx3 expression to improve mitochondrial dysfunction and block senescence in AT2 cells, revealing a potential novel therapeutic strategy for pulmonary fibrosis. Idiopathic pulmonary fibrosis is still not fully understood, and effective treatments are scarce. This study investigates the role of a protein, YAP1, in lung fibrosis. Researchers used mice and human lung samples to study how YAP1 influences lung cell aging and lung structure. The findings showed that increasing YAP1 levels in alveolar type II cells reduced lung fibrosis by improving the function of mitochondria and decreasing cell aging. Specifically, YAP1 worked through a pathway involving a molecule, Prdx3. However, reducing YAP1 levels worsened lung fibrosis. The researchers suggest that enhancing YAP1 activity in lung cells could be a potential treatment for lung fibrosis. This method targets lung cell aging and promotes healthy lung tissue repair. The results pave the way for developing treatments for IPF and possibly other similar lung diseases. This summary was initially drafted using artificial intelligence, then revised and fact-checked by the author.
YAP1 通过靶向 Prdx3 抑制肺泡上皮细胞的衰老,从而缓解肺纤维化。
肺泡II型(AT2)细胞的衰老阻碍了肺上皮细胞的自我修复,并在特发性肺纤维化(IPF)中导致肺损伤。是相关蛋白1(YAP1)对细胞生长和器官发育至关重要;然而,YAP1在肺纤维化过程中在AT2细胞中的作用仍不清楚。研究发现,肺纤维化患者的 AT2 细胞中 YAP1 表达下调。AT2 细胞中 YAP1 的缺失会导致肺损伤、细胞外基质(ECM)沉积加剧和肺功能恶化。相反,在 AT2 细胞中过表达 YAP1 可促进肺泡再生、减轻肺纤维化并改善肺功能。此外,在体内和体外,过表达 YAP1 可减轻博莱霉素(BLM)诱导的肺泡上皮细胞衰老。此外,YAP1通过与TEAD1直接相互作用,促进过氧化物酶3(Prdx3)的表达。强制表达 Prdx3 可抑制 BLM 处理的 MLE-12 细胞的衰老并改善线粒体功能障碍,而耗尽 Prdx3 则会部分削弱 YAP1 的保护作用。此外,过表达 Prdx3 可促进损伤肺的自我修复并减少 ECM 沉积,而沉默 Prdx3 则会削弱 YAP1 的抗纤维化作用。总之,这项研究表明,YAP1通过调节Prdx3的表达来改善线粒体功能障碍和阻断AT2细胞的衰老,从而缓解肺损伤和肺纤维化,为肺纤维化的治疗提供了一种潜在的新型治疗策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Experimental and Molecular Medicine
医学-生化与分子生物学
CiteScore
19.50
自引率
0.80%
发文量
166
审稿时长
3 months
期刊介绍:
Experimental & Molecular Medicine (EMM) stands as Korea's pioneering biochemistry journal, established in 1964 and rejuvenated in 1996 as an Open Access, fully peer-reviewed international journal. Dedicated to advancing translational research and showcasing recent breakthroughs in the biomedical realm, EMM invites submissions encompassing genetic, molecular, and cellular studies of human physiology and diseases. Emphasizing the correlation between experimental and translational research and enhanced clinical benefits, the journal actively encourages contributions employing specific molecular tools. Welcoming studies that bridge basic discoveries with clinical relevance, alongside articles demonstrating clear in vivo significance and novelty, Experimental & Molecular Medicine proudly serves as an open-access, online-only repository of cutting-edge medical research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


