From inflammation to bone formation: the intricate role of neutrophils in skeletal muscle injury and traumatic heterotopic ossification
IF 9.5
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
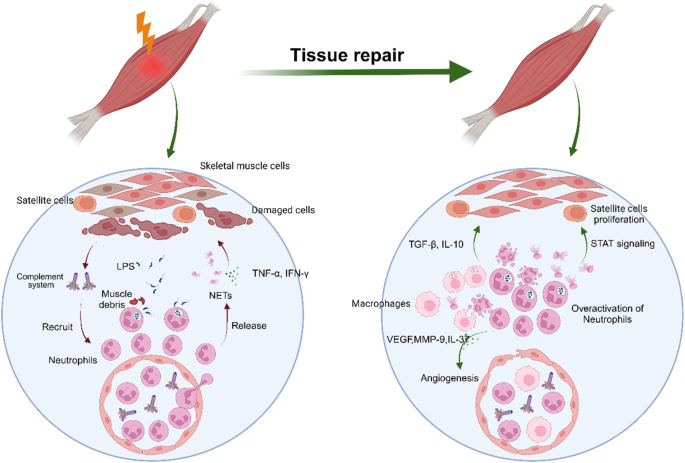
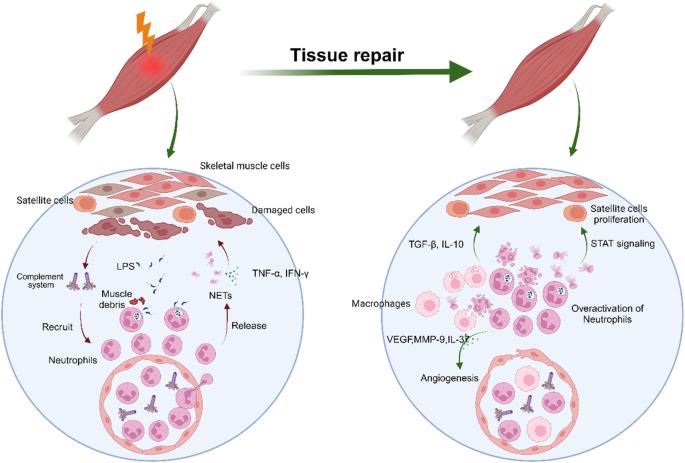
Neutrophils are emerging as an important player in skeletal muscle injury and repair. Neutrophils accumulate in injured tissue, thus releasing inflammatory factors, proteases and neutrophil extracellular traps (NETs) to clear muscle debris and pathogens when skeletal muscle is damaged. During the process of muscle repair, neutrophils can promote self-renewal and angiogenesis in satellite cells. When neutrophils are abnormally overactivated, neutrophils cause collagen deposition, functional impairment of satellite cells, and damage to the skeletal muscle vascular endothelium. Heterotopic ossification (HO) refers to abnormal bone formation in soft tissue. Skeletal muscle injury is one of the main causes of traumatic HO (tHO). Neutrophils play a pivotal role in activating BMPs and TGF-β signals, thus promoting the differentiation of mesenchymal stem cells and progenitor cells into osteoblasts or osteoclasts to facilitate HO. Furthermore, NETs are specifically localized at the site of HO, thereby accelerating the formation of HO. Additionally, the overactivation of neutrophils contributes to the disruption of immune homeostasis to trigger HO. An understanding of the diverse roles of neutrophils will not only provide more information on the pathogenesis of skeletal muscle injury for repair and HO but also provides a foundation for the development of more efficacious treatment modalities for HO. Skeletal muscle, the body’s most common tissue, often gets injured and lacks highly effective treatments. This review investigates the complex relationship between skeletal muscle and neutrophils during injury and healing. Researchers study how neutrophils can both worsen muscle damage and assist in tissue repair. It looks at how neutrophil activity affects muscle repair, explaining the processes of inflammation, tissue regeneration, and the factors causing heterotopic ossification. It also emphasizes the significance of controlling neutrophil activity for effective muscle healing and avoiding complications. Key findings show that neutrophils are crucial in both harming and repairing skeletal muscle. Overactive neutrophils can cause extended inflammation, hindering the healing process, while controlled activity aids tissue regeneration. The researchers suggest that focusing on neutrophil activity could be a promising method for treating muscle injuries and preventing heterotopic ossification. This summary was initially drafted using artificial intelligence, then revised and fact-checked by the author.
从炎症到骨形成:中性粒细胞在骨骼肌损伤和创伤性异位骨化中的复杂作用。
中性粒细胞正在成为骨骼肌损伤和修复的重要参与者。当骨骼肌受损时,中性粒细胞会聚集在受伤组织中,从而释放炎症因子、蛋白酶和中性粒细胞胞外捕获器(NET),清除肌肉碎片和病原体。在肌肉修复过程中,中性粒细胞可促进卫星细胞的自我更新和血管生成。当中性粒细胞异常过度激活时,中性粒细胞会导致胶原蛋白沉积、卫星细胞功能受损以及骨骼肌血管内皮受损。异位骨化(HO)是指软组织中异常的骨形成。骨骼肌损伤是创伤性异位骨化(tHO)的主要原因之一。中性粒细胞在激活BMPs和TGF-β信号方面起着关键作用,从而促进间充质干细胞和祖细胞分化为成骨细胞或破骨细胞,促进HO的形成。此外,NETs会特异性地定位在HO部位,从而加速HO的形成。此外,中性粒细胞的过度激活也会破坏免疫平衡,从而引发HO。了解中性粒细胞的不同作用不仅能为骨骼肌损伤修复和HO的发病机制提供更多信息,还能为开发更有效的HO治疗方法奠定基础。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Experimental and Molecular Medicine
医学-生化与分子生物学
CiteScore
19.50
自引率
0.80%
发文量
166
审稿时长
3 months
期刊介绍:
Experimental & Molecular Medicine (EMM) stands as Korea's pioneering biochemistry journal, established in 1964 and rejuvenated in 1996 as an Open Access, fully peer-reviewed international journal. Dedicated to advancing translational research and showcasing recent breakthroughs in the biomedical realm, EMM invites submissions encompassing genetic, molecular, and cellular studies of human physiology and diseases. Emphasizing the correlation between experimental and translational research and enhanced clinical benefits, the journal actively encourages contributions employing specific molecular tools. Welcoming studies that bridge basic discoveries with clinical relevance, alongside articles demonstrating clear in vivo significance and novelty, Experimental & Molecular Medicine proudly serves as an open-access, online-only repository of cutting-edge medical research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


