Development of a novel testis-on-a-chip that demonstrates reciprocal crosstalk between Sertoli and Leydig cells in testicular tissue
IF 9.5
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
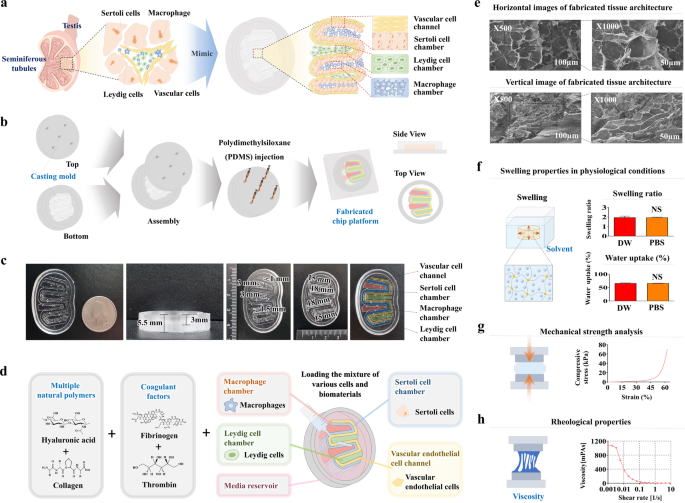
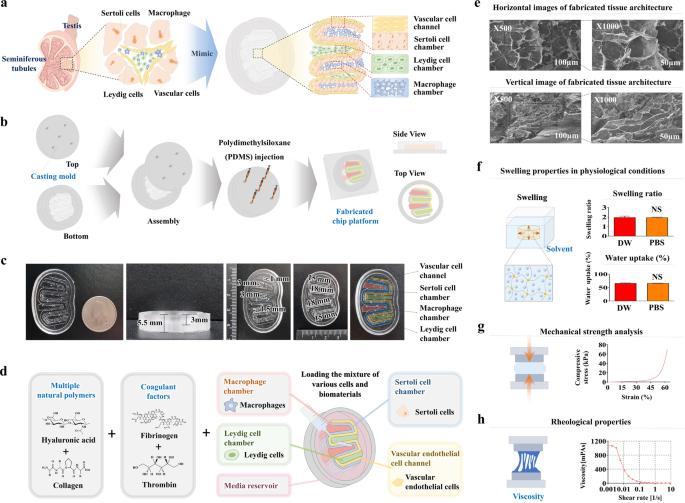
The reciprocal crosstalk between testicular Sertoli and Leydig cells plays a vital role in supporting germ cell development and maintaining testicular characteristics and spermatogenesis. Conventional 2D and the recent 3D assay systems fail to accurately replicate the dynamic interactions between these essential endocrine cells. Furthermore, most in vitro testicular tissue models lack the ability to capture the complex multicellular nature of the testis. To address these limitations, we developed a 3D multicellular testis-on-a-chip platform that effectively demonstrates the reciprocal crosstalk between Sertoli cells and the adjacent Leydig cells while incorporating various human testicular tissue constituent cells and various natural polymers infused with blood coagulation factors. Additionally, we identified SERPINB2 as a biomarker of male reproductive toxicity that is activated in both Sertoli and Leydig cells upon exposure to various toxicants. Leveraging this finding, we designed a fluorescent reporter-conjugated toxic biomarker detection system that enables both an intuitive and quantitative assessment of material toxicity by measuring the converted fluorescence intensity. By integrating this fluorescent reporter system into the Sertoli and Leydig cells within our 3D multicellular chip platform, we successfully developed a testis-on-chip model that can be utilized to evaluate the male reproductive toxicity of potential drug candidates. This innovative approach holds promise for advancing toxicity screening and reproductive research. Spermatogenesis, or sperm creation, happens in the testis and involves various cells, including Sertoli and Leydig cells. However, traditional single-cell-based 2D assay models (tests that measure the presence of a substance) don’t accurately show the complex interactions between these cells. To solve this, scientists created a ‘human testis-on-a-chip’ platform that imitates the complex cell interactions and hormone communication of seminiferous tubules (small tubes) in the testis. The chip was made using polydimethylsiloxane (a type of silicone) and included multiple testicular tissue cells. The scientists found that the chip could keep the cells alive and active for up to 28 days. Also, the chip was able to produce hormones and respond to hormonal stimulation. This study provides a useful tool for studying male reproductive biology and testing potential drugs. This summary was initially drafted using artificial intelligence, then revised and fact-checked by the author.
开发新型睾丸芯片,展示睾丸组织中 Sertoli 细胞和 Leydig 细胞之间的相互串扰。
睾丸Sertoli细胞和Leydig细胞之间的相互交织在支持生殖细胞发育、维持睾丸特征和精子生成方面起着至关重要的作用。传统的二维和最新的三维检测系统无法准确复制这些重要内分泌细胞之间的动态相互作用。此外,大多数体外睾丸组织模型都无法捕捉睾丸复杂的多细胞特性。为了解决这些局限性,我们开发了一种三维多细胞睾丸芯片平台,它能有效地展示 Sertoli 细胞和邻近的 Leydig 细胞之间的相互串扰,同时结合了各种人类睾丸组织成分细胞和注入血液凝固因子的各种天然聚合物。此外,我们还发现 SERPINB2 是男性生殖毒性的生物标志物,当暴露于各种有毒物质时,Sertoli 和 Leydig 细胞中的 SERPINB2 都会被激活。利用这一发现,我们设计了一种荧光报告物共轭毒性生物标记物检测系统,通过测量转换后的荧光强度,对物质毒性进行直观和定量评估。通过将这种荧光报告系统集成到我们的三维多细胞芯片平台中的 Sertoli 细胞和 Leydig 细胞中,我们成功开发出了一种睾丸芯片模型,可用于评估潜在候选药物的男性生殖毒性。这种创新方法有望推动毒性筛选和生殖研究。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Experimental and Molecular Medicine
医学-生化与分子生物学
CiteScore
19.50
自引率
0.80%
发文量
166
审稿时长
3 months
期刊介绍:
Experimental & Molecular Medicine (EMM) stands as Korea's pioneering biochemistry journal, established in 1964 and rejuvenated in 1996 as an Open Access, fully peer-reviewed international journal. Dedicated to advancing translational research and showcasing recent breakthroughs in the biomedical realm, EMM invites submissions encompassing genetic, molecular, and cellular studies of human physiology and diseases. Emphasizing the correlation between experimental and translational research and enhanced clinical benefits, the journal actively encourages contributions employing specific molecular tools. Welcoming studies that bridge basic discoveries with clinical relevance, alongside articles demonstrating clear in vivo significance and novelty, Experimental & Molecular Medicine proudly serves as an open-access, online-only repository of cutting-edge medical research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


