Activation of the osteoblastic HIF-1α pathway partially alleviates the symptoms of STZ-induced type 1 diabetes mellitus via RegIIIγ
IF 9.5
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
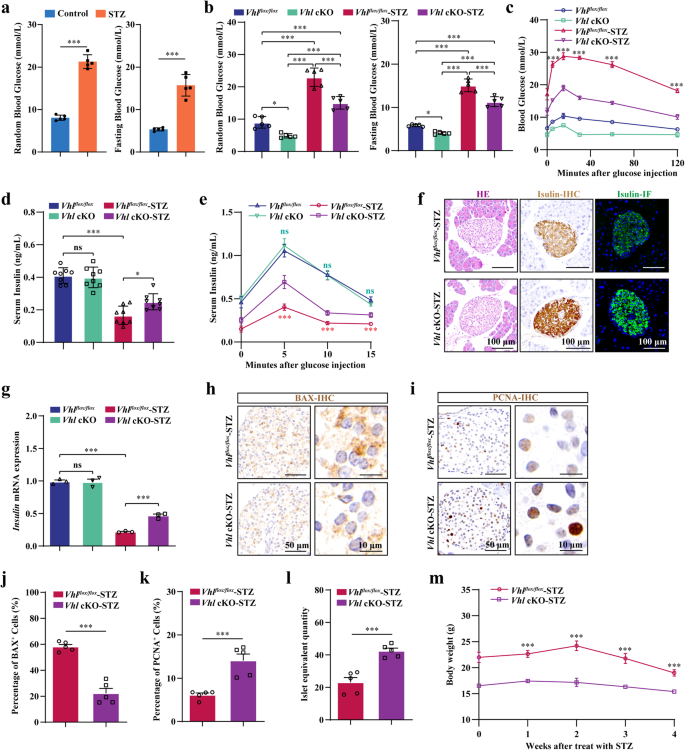
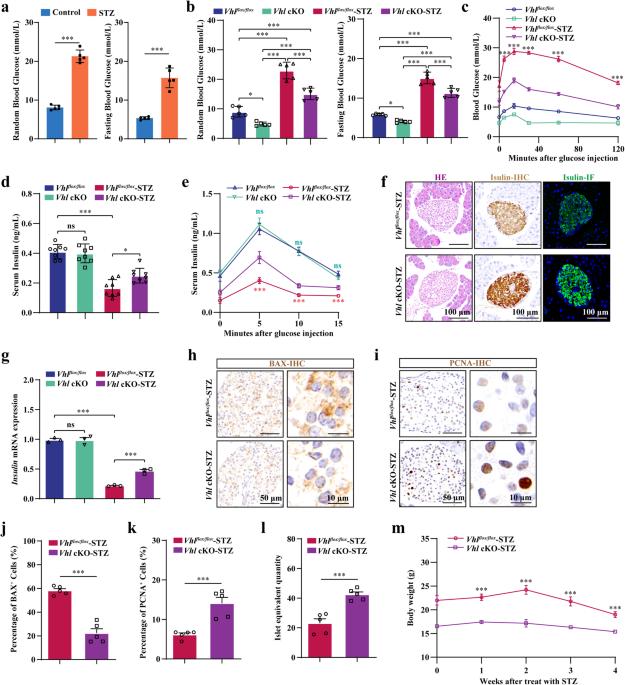
The hypoxia-inducible factor-1α (HIF-1α) pathway coordinates skeletal bone homeostasis and endocrine functions. Activation of the HIF-1α pathway increases glucose uptake by osteoblasts, which reduces blood glucose levels. However, it is unclear whether activating the HIF-1α pathway in osteoblasts can help normalize glucose metabolism under diabetic conditions through its endocrine function. In addition to increasing bone mass and reducing blood glucose levels, activating the HIF-1α pathway by specifically knocking out Von Hippel‒Lindau (Vhl) in osteoblasts partially alleviated the symptoms of streptozotocin (STZ)-induced type 1 diabetes mellitus (T1DM), including increased glucose clearance in the diabetic state, protection of pancreatic β cell from STZ-induced apoptosis, promotion of pancreatic β cell proliferation, and stimulation of insulin secretion. Further screening of bone-derived factors revealed that islet regeneration-derived protein III gamma (RegIIIγ) is an osteoblast-derived hypoxia-sensing factor critical for protection against STZ-induced T1DM. In addition, we found that iminodiacetic acid deferoxamine (SF-DFO), a compound that mimics hypoxia and targets bone tissue, can alleviate symptoms of STZ-induced T1DM by activating the HIF-1α-RegIIIγ pathway in the skeleton. These data suggest that the osteoblastic HIF-1α-RegIIIγ pathway is a potential target for treating T1DM. The skeleton isn’t just for support, it also helps control body functions. This research looked at how a specific process in bone-forming cells, called the hypoxia-inducible factor-1 alpha (HIF-1α) pathway, affects sugar breakdown and diabetes. The scientists discovered that triggering this process in these cells can help manage sugar levels in diabetes through a protein named RegIIIγ. They also found that a substance named SF-DFO, which imitates low oxygen conditions and focuses on bone tissue, can somewhat ease type 1 diabetes symptoms by triggering the HIF-1α-RegIIIγ process in the skeleton. This implies that this specific process in bone-forming cells could be a possible treatment for type 1 diabetes. This summary was initially drafted using artificial intelligence, then revised and fact-checked by the author.
通过 RegIIIγ 激活成骨细胞 HIF-1α 通路可部分缓解 STZ 诱导的 1 型糖尿病的症状。
缺氧诱导因子-1α(HIF-1α)通路协调着骨骼的骨稳态和内分泌功能。激活 HIF-1α 通路可增加成骨细胞对葡萄糖的吸收,从而降低血糖水平。然而,激活成骨细胞中的 HIF-1α 通路是否能通过其内分泌功能帮助糖尿病患者实现葡萄糖代谢正常化,目前尚不清楚。通过特异性敲除成骨细胞中的Von Hippel-Lindau(Vhl)来激活HIF-1α通路,除了能增加骨量和降低血糖水平外,还能部分缓解链脲佐菌素(STZ)诱导的1型糖尿病(T1DM)的症状,包括增加糖尿病状态下的葡萄糖清除率、保护胰腺β细胞免受STZ诱导的凋亡、促进胰腺β细胞增殖以及刺激胰岛素分泌。对骨源性因子的进一步筛选发现,胰岛再生衍生蛋白Ⅲγ(RegⅢγ)是一种成骨细胞衍生的低氧传感因子,对保护STZ诱导的T1DM至关重要。此外,我们还发现亚氨基二乙酸去氧胺(SF-DFO)是一种模拟缺氧并靶向骨组织的化合物,它能通过激活骨骼中的 HIF-1α-RegIIIγ 通路来缓解 STZ 诱导的 T1DM 症状。这些数据表明,成骨细胞的 HIF-1α-RegIIIγ 通路是治疗 T1DM 的潜在靶点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Experimental and Molecular Medicine
医学-生化与分子生物学
CiteScore
19.50
自引率
0.80%
发文量
166
审稿时长
3 months
期刊介绍:
Experimental & Molecular Medicine (EMM) stands as Korea's pioneering biochemistry journal, established in 1964 and rejuvenated in 1996 as an Open Access, fully peer-reviewed international journal. Dedicated to advancing translational research and showcasing recent breakthroughs in the biomedical realm, EMM invites submissions encompassing genetic, molecular, and cellular studies of human physiology and diseases. Emphasizing the correlation between experimental and translational research and enhanced clinical benefits, the journal actively encourages contributions employing specific molecular tools. Welcoming studies that bridge basic discoveries with clinical relevance, alongside articles demonstrating clear in vivo significance and novelty, Experimental & Molecular Medicine proudly serves as an open-access, online-only repository of cutting-edge medical research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


