Adult cardiomyocytes-derived EVs for the treatment of cardiac fibrosis
Abstract
Cardiac fibrosis is a common pathological feature of cardiovascular diseases that arises from the hyperactivation of fibroblasts and excessive extracellular matrix (ECM) deposition, leading to impaired cardiac function and potentially heart failure or arrhythmia. Extracellular vesicles (EVs) released by cardiomyocytes (CMs) regulate various physiological functions essential for myocardial homeostasis, which are disrupted in cardiac disease. Therefore, healthy CM-derived EVs represent a promising cell-free therapy for the treatment of cardiac fibrosis.
To this end, we optimized the culture conditions of human adult CMs to obtain a large yield of EVs without compromising cellular integrity by using a defined combination of small molecules. EVs were isolated by ultracentrifugation, and their characteristics were analysed. Finally, their effect on fibrosis was tested.
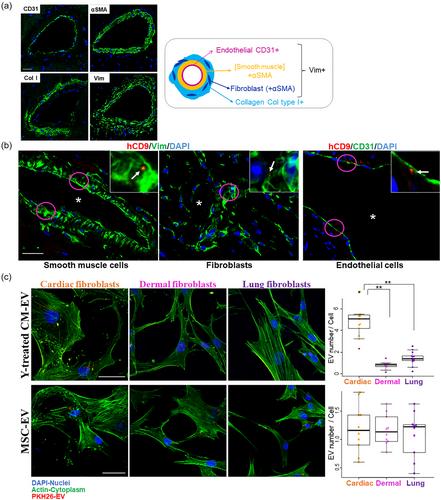
Treatment of TGFβ-activated human cardiac fibroblasts with EVs derived from CMs using our culture system resulted in a decrease in fibroblast activation markers and ECM accumulation. The rescued phenotype was associated with specific EV cargo, including multiple myocyte-specific and antifibrotic microRNAs, although their effect individually was not as effective as the EV treatment. Notably, pathway analysis showed that EV treatment reverted the transcription of activated fibroblasts and decreased several signalling pathways, including MAPK, mTOR, JAK/STAT, TGFβ, and PI3K/Akt, all of which are involved in fibrosis development. Intracardiac injection of CM-derived EVs in an animal model of cardiac fibrosis reduced fibrotic area and increased angiogenesis, which correlated with improved cardiac function.
These findings suggest that EVs derived from human adult CMs may offer a targeted and effective treatment for cardiac fibrosis, owing to their antifibrotic properties and the specificity of cargo.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


