Evaluation of serial QuantiFERON-TB Gold in tube test results and tuberculosis infection status in patients with psoriasis receiving anti-IL-17 treatment (secukinumab and ixekizumab): Real-world data from a tuberculosis-endemic country
Abstract
Background
In comparison with TNF-α inhibitors, anti-IL-17A agents are considered to have a lower risk of active tuberculosis (TB) or latent TB infection (LTBI) reactivation.
Methods
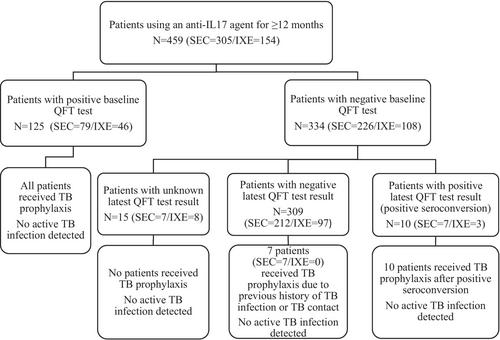
In this study, we aimed to evaluate the TB infection status and serial QuantiFERON-TB-Gold in tube test (QFT) results of psoriasis patients using IL-17 inhibitors (secukinumab [SEC] and ixekizumab [IXE]) in a real-world setting from a tuberculosis-endemic country. Patients who used an anti-IL-17 agent for at least 3 months in our follow-up were included in the study. Patients' clinical and demographic features, baseline QFT results and latest QFT results (if any), and TB infection status were noted from the past medical records.
Results
A total of 717 patients, of whom 333 (46.4%) were female, were included in the study. The cumulative exposure time to an anti-IL-17 agent was 14,147 patient-months, 9743 patient-months for SEC and 4404 patient-months for IXE. Also, 459 (SEC = 305/IXE = 154) patients used an anti-IL-17 agent for ≥ 12 months. Of these, 125 had positive baseline QFT results. In all, 334 had negative baseline QFT results. The latest QFT result of 309 was also negative (persistent seronegative group). During follow-up, the QFT results of 10 patients changed from negative to positive (positive seroconversion group). Seven of them were using SEC and three were using IXE, respectively. No case of active TB infection was detected.
Conclusion
In our study, the positive seroconversion rate of 10/334 seems high, but this did not translate to active disease. However, closer monitoring may be required, especially in patients with advanced age, the presence of PsA, long disease duration and long anti-IL-17 treatment duration.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


