Leveraging in situ N-tosylhydrazones as diazo surrogates for efficient access to pyrazolo-[1,5-c]quinazolinone derivatives†
IF 2.9
3区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
We developed a transition metal-free methodology for the construction of pyrazoloquinazolinone derivatives. The strategy involves a one-pot reaction wherein the N-tosylhydrazone and its corresponding diazo derivative are generated in situ, followed by an intramolecular 1,3-dipolar cycloaddition–ring expansion to provide the pyrazolo-[1,5-c]quinazolinone motif. This approach enables straightforward access to a diverse range of highly functionalized N-heterocyclic compounds in good yields (up to 92%).
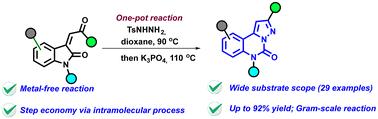

利用原位 N-对甲苯磺酸肼作为重氮代用品,高效获取吡唑-[1,5-c]喹唑啉酮衍生物。
我们开发了一种用于构建吡唑喹唑啉酮衍生物的无过渡金属方法。该策略涉及一个一锅反应,其中 N-对甲苯磺酰腙及其相应的重氮衍生物在原位生成,然后进行分子内 1,3-二极环化-扩环反应,以提供吡唑并[1,5-c]喹唑啉酮基团。通过这种方法,可以直接获得各种高官能度的 N-杂环化合物,而且产率高(高达 92%)。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic & Biomolecular Chemistry
化学-有机化学
CiteScore
5.50
自引率
9.40%
发文量
1056
审稿时长
1.3 months
期刊介绍:
The international home of synthetic, physical and biomolecular organic chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


