Electrochemical synthesis of β-difluoromethylamide compounds by N-benzenesulfonylacrylamide with difluorine reagents†
IF 4.2
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
A mild and efficient electrochemical method for radical addition, cyclization, and migration reaction was described in this work. A difluoromethyl radical was produced by anodizing CF2HSO2Na. The resulting product was then added to olefin, underwent Smiles cyclization, and migrated to form β-difluoromethamide compounds after the release of SO2. The process was free from metals and catalysts, gram-grade, and resistant to a variety of electron-rich substrates.
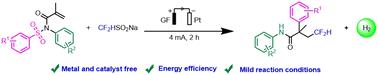
N-苯磺酰丙烯酰胺与二氟试剂电化学合成 β-二氟甲基酰胺化合物
这项工作描述了一种用于自由基加成、环化和迁移反应的温和高效的电化学方法。通过阳极氧化 CF2HSO2Na 生成二氟甲基自由基。然后将生成物添加到烯烃中,进行 Smiles 环化反应,并在释放 SO2 后迁移生成 β-二氟甲酰胺化合物。该工艺不含金属和催化剂,是克级工艺,对多种富电子底物具有耐受性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Communications
化学-化学综合
CiteScore
8.60
自引率
4.10%
发文量
2705
审稿时长
1.4 months
期刊介绍:
ChemComm (Chemical Communications) is renowned as the fastest publisher of articles providing information on new avenues of research, drawn from all the world''s major areas of chemical research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


