Modular assembly of arenes, ethylene and heteroarenes for the synthesis of 1,2-arylheteroaryl ethanes
IF 19.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
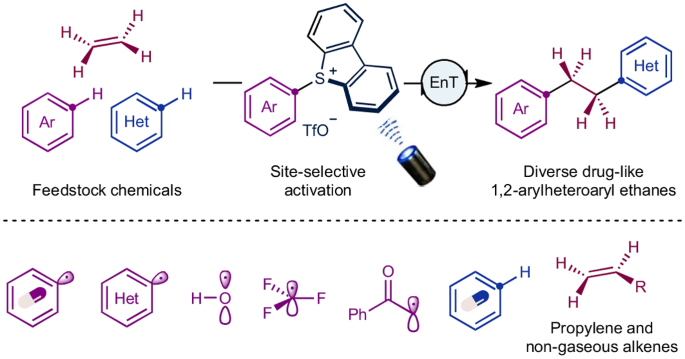
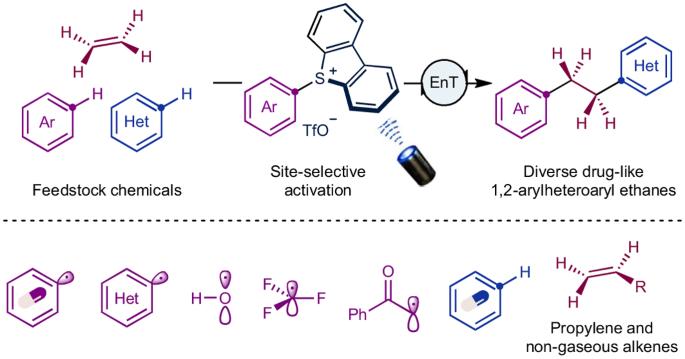
The 1,2-arylheteroaryl ethane motif stands as a privileged scaffold with promising implications in drug discovery. Conventional de novo syntheses of these molecules have relied heavily on pre-functionalized synthons, entailing harsh conditions and multi-step processes. Here, to address these limitations, we present a modular approach for the direct synthesis of 1,2-arylheteroaryl ethanes using feedstock chemicals, including ethylene, arenes and heteroarenes. We disclosed a photo triplet-energy-transfer-initiated radical cascade process, leveraging homolytic cleavage of C–S bonds in aryl sulfonium salts as the key step to access aryl radicals with excellent regioselectivity. This method allows for rapid structural diversification of bioactive molecules, showcasing excellent functional group tolerance and streamlining the synthesis of bioactive compounds and their derivatives. Furthermore, our approach can be extended to propylene, non-gaseous terminal alkenes and various other electrophilic radical precursors, including heteroaryl radicals, hydroxyl radicals, trifluoromethyl radicals and α-carbonyl alkyl radicals. This study highlights the significance of radical polarity matching in designing selective multi-component couplings. De novo syntheses of 1,2-arylheteroaryl ethanes, key scaffolds in drug discovery, are challenging, typically relying on pre-functionalized synthons, harsh conditions and multi-step processes. Now a modular assembly of arenes, ethylene and heteroarenes yields diverse drug-like 1,2-arylheteroaryl ethanes, highlighting the importance of radical polarity matching in selective multi-component couplings.
烷、乙烯和杂环戊烯的模块化组装,用于合成 1,2-芳基杂芳基乙烷
1,2-芳基杂芳基乙烷基团是一种特殊的支架,在药物发现方面具有广阔的前景。这些分子的传统从头合成在很大程度上依赖于预官能化合成物,需要苛刻的条件和多步过程。为了解决这些局限性,我们在此提出了一种模块化方法,利用乙烯、炔烃和杂烯等原料化学品直接合成 1,2-芳基芳香族乙烷。我们披露了一种由光三重能量转移引发的自由基级联过程,利用芳基锍盐中 C-S 键的均聚裂解作为关键步骤,以优异的区域选择性获得芳基自由基。这种方法可以快速实现生物活性分子结构的多样化,显示出优异的官能团耐受性,并简化了生物活性化合物及其衍生物的合成过程。此外,我们的方法还可扩展到丙烯、非气态末端烯和其他各种亲电自由基前体,包括杂芳基自由基、羟基自由基、三氟甲基自由基和 α-羰基烷基自由基。这项研究强调了自由基极性匹配在设计选择性多组分偶联反应中的重要性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature chemistry
化学-化学综合
CiteScore
29.60
自引率
1.40%
发文量
226
审稿时长
1.7 months
期刊介绍:
Nature Chemistry is a monthly journal that publishes groundbreaking and significant research in all areas of chemistry. It covers traditional subjects such as analytical, inorganic, organic, and physical chemistry, as well as a wide range of other topics including catalysis, computational and theoretical chemistry, and environmental chemistry.
The journal also features interdisciplinary research at the interface of chemistry with biology, materials science, nanotechnology, and physics. Manuscripts detailing such multidisciplinary work are encouraged, as long as the central theme pertains to chemistry.
Aside from primary research, Nature Chemistry publishes review articles, news and views, research highlights from other journals, commentaries, book reviews, correspondence, and analysis of the broader chemical landscape. It also addresses crucial issues related to education, funding, policy, intellectual property, and the societal impact of chemistry.
Nature Chemistry is dedicated to ensuring the highest standards of original research through a fair and rigorous review process. It offers authors maximum visibility for their papers, access to a broad readership, exceptional copy editing and production standards, rapid publication, and independence from academic societies and other vested interests.
Overall, Nature Chemistry aims to be the authoritative voice of the global chemical community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


