COMBAT-MS: A Population-Based Observational Cohort Study Addressing the Benefit–Risk Balance of Multiple Sclerosis Therapies Compared with Rituximab
Abstract
Objective
To assess comparative effectiveness, safety, and tolerability of off-label rituximab, compared with frequently used therapies approved for multiple sclerosis (MS).
Methods
A Swedish cohort study of persons with relapsing–remitting MS, age 18 to 75 years at inclusion and with a first therapy start or a first therapy switch between 2011 and 2018. Low-dose rituximab was compared with MS-approved therapies. Primary outcomes were proportions with 12 months confirmed disability worsening and change in MS Impact Scale-29 (MSIS-29) scores, respectively. Secondary endpoints included relapses, therapy discontinuation, and serious adverse events. Analyses used an intention-to-treat approach and were adjusted for demographics, MS features, and health characteristics.
Results
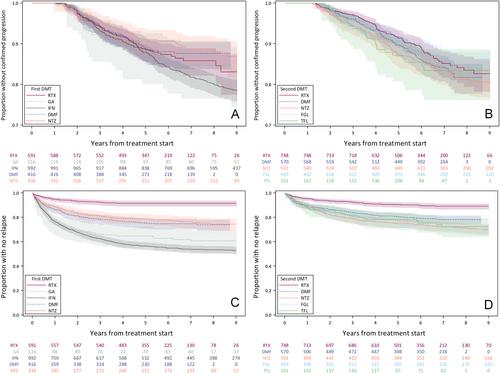
We included 2,449 participants as first therapy start and 2,463 as first therapy switch. Proportions with disability worsening at 3 years were 9.1% for rituximab as first therapy and 5.1% after therapy switch, with no differences to MS-approved comparators. Worsening on rituximab was mostly independent of relapses. MSIS-29 with rituximab at 3 years improved by 1.3/8.4 points (physical/psychological) for first disease-modifying therapy (DMT) and 0.4/3.6 for DMT switch, and was mostly similar across therapies. Rituximab had lower relapse rates and higher therapy persistence in both groups. The rate of hospital-treated infections was higher with rituximab after a therapy switch, but not as a first therapy.
Interpretation
This population-based real-world cohort study found low rates of disability progression, mostly independent of relapses, and without significant differences between rituximab and MS-approved comparators. Rituximab led to lower rates of inflammatory activity and higher treatment persistence, but was associated with an increased rate of serious infections. ANN NEUROL 2024;96:678–693

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


