Metal-Hydride C-C Cross-Coupling of Alkenes Through a Double Outer-Sphere Mechanism.
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
The Journal of Organic Chemistry
Pub Date : 2024-11-15
Epub Date: 2024-06-26
DOI:10.1021/acs.joc.4c00260
引用次数: 0
Abstract
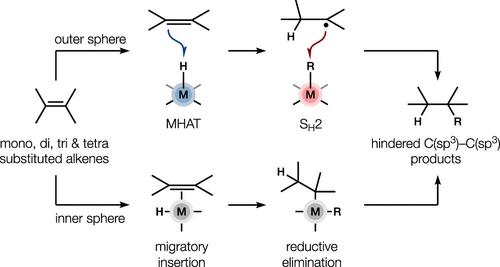
This Synopsis covers recent reports of metal-catalyzed alkene functionalizations that likely involve iterative outer-sphere reactions in which the substrate reacts directly with a metal ligand instead of with the metal center itself. Traditional metal hydride-catalyzed alkene functionalizations involve this latter pathway whereby the alkene forms part of the metal ligand sphere (i.e. an inner-sphere reaction). In contrast, alkenes do not ligate the metal in so-called outer-sphere reactions and instead react with a metal ligand. These transformations have proved crucial for the synthesis of high fraction sp3 (Fsp3) targets, especially in hindered fragment couplings of relevance to natural product space.
通过双外球机制实现烯烃的金属氢化物 C-C 交叉偶联
本简介涵盖最近有关金属催化烯官能化的报道,这些官能化可能涉及迭代外球反应,其中底物直接与金属配体而不是金属中心本身发生反应。传统的金属氢化物催化烯官能化涉及后一种途径,烯形成金属配体球的一部分(即内球反应)。相反,在所谓的外球反应中,烯并不与金属连接,而是与金属配体发生反应。事实证明,这些转化对于合成高分sp3(Fsp3)目标物至关重要,尤其是在与天然产物空间相关的受阻片段偶联方面。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
The Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


