Ferroptosis-like cell death promotes and prolongs inflammation in Drosophila
IF 17.3
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
Abstract
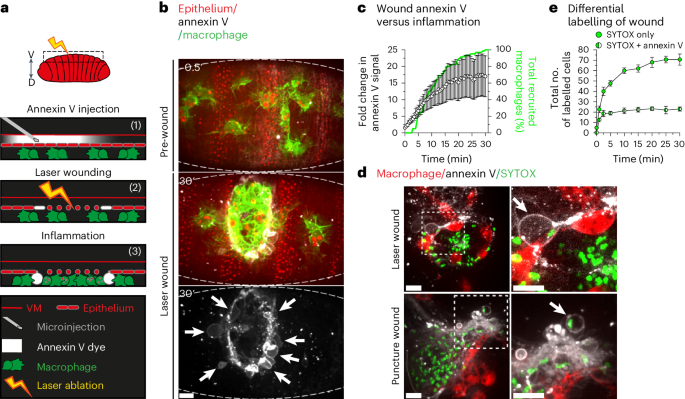
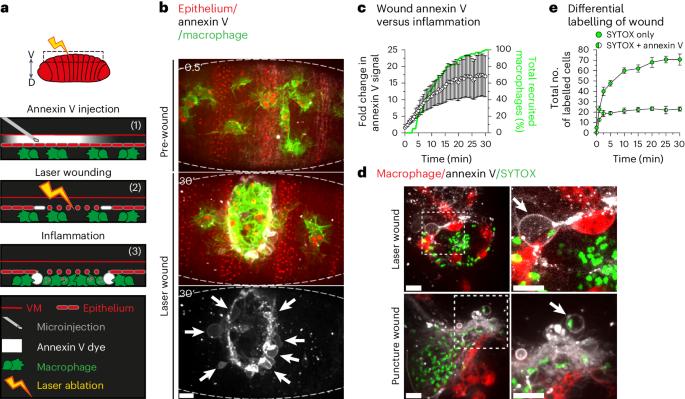
Ferroptosis is a distinct form of necrotic cell death caused by overwhelming lipid peroxidation, and emerging evidence indicates a major contribution to organ damage in multiple pathologies. However, ferroptosis has not yet been visualized in vivo due to a lack of specific probes, which has severely limited the study of how the immune system interacts with ferroptotic cells and how this process contributes to inflammation. Consequently, whether ferroptosis has a physiological role has remained a key outstanding question. Here we identify a distinct, ferroptotic-like, necrotic cell death occurring in vivo during wounding of the Drosophila embryo using live imaging. We further demonstrate that macrophages rapidly engage these necrotic cells within the embryo but struggle to engulf them, leading to prolonged, frustrated phagocytosis and frequent corpse disintegration. Conversely, suppression of the ferroptotic programme during wounding delays macrophage recruitment to the injury site, pointing to conflicting roles for ferroptosis during inflammation in vivo. Davidson et al. visualize ferroptosis-like cell death using three-colour live imaging in vivo and demonstrate its role in triggering macrophage recruitment but delaying resolution of inflammation during wounding in the Drosophila embryo.
类铁蛋白酶细胞死亡促进并延长果蝇的炎症反应
铁蜕变是一种独特的细胞坏死形式,由大量脂质过氧化引起,新出现的证据表明,铁蜕变在多种病症的器官损伤中起着重要作用。然而,由于缺乏特异性探针,铁蜕变尚未在体内可视化,这严重限制了对免疫系统如何与铁蜕变细胞相互作用以及这一过程如何导致炎症的研究。因此,嗜铁细胞是否具有生理作用仍然是一个悬而未决的关键问题。在这里,我们利用活体成像技术发现了果蝇胚胎受伤时体内发生的一种独特的、类似于嗜铁细胞的坏死细胞。我们进一步证明,巨噬细胞在胚胎内迅速与这些坏死细胞接触,但却难以将其吞噬,导致吞噬过程延长、受挫和尸体频繁解体。相反,在创伤过程中抑制铁凋亡程序会延迟巨噬细胞被招募到损伤部位,这表明铁凋亡在体内炎症过程中扮演着相互冲突的角色。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Cell Biology
生物-细胞生物学
CiteScore
28.40
自引率
0.90%
发文量
219
审稿时长
3 months
期刊介绍:
Nature Cell Biology, a prestigious journal, upholds a commitment to publishing papers of the highest quality across all areas of cell biology, with a particular focus on elucidating mechanisms underlying fundamental cell biological processes. The journal's broad scope encompasses various areas of interest, including but not limited to:
-Autophagy
-Cancer biology
-Cell adhesion and migration
-Cell cycle and growth
-Cell death
-Chromatin and epigenetics
-Cytoskeletal dynamics
-Developmental biology
-DNA replication and repair
-Mechanisms of human disease
-Mechanobiology
-Membrane traffic and dynamics
-Metabolism
-Nuclear organization and dynamics
-Organelle biology
-Proteolysis and quality control
-RNA biology
-Signal transduction
-Stem cell biology
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


