High-pressure synthesis of Ruddlesden–Popper nitrides
IF 19.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Layered perovskites with Ruddlesden–Popper-type structures are fundamentally important for low-dimensional properties, for example, photovoltaic hybrid iodides and superconducting copper oxides. Many such halides and oxides are known, but analogous nitrides are difficult to stabilize due to the high cation oxidation states required to balance the anion charges. Here we report the high-pressure synthesis of three single-layer Ruddlesden–Popper (K2NiF4 type) nitrides—Pr2ReN4, Nd2ReN4 and Ce2TaN4—along with their structural characterization and properties. The R2ReN4 materials (R = Pr and Nd) are metallic, and Nd2ReN4 has a ferromagnetic Nd3+ spin order below 15 K. Thermal decomposition gives R2ReN3 with a Peierls-type distortion and chains of Re–Re multiply bonded dimers. Ce2TaN4 has a structural transition driven by octahedral tilting, with local distortions and canted magnetic Ce3+ order evidencing two-dimensional Ce3+/Ce4+ charge ordering correlations. Our work demonstrates that Ruddlesden–Popper nitrides with varied structural, electronic and magnetic properties can be prepared from high-pressure synthesis, opening the door to related layered nitride materials. Nitrogen-rich Ruddlesden–Popper nitrides are notoriously difficult to stabilize. Now a high-pressure high-temperature synthesis method has enabled the preparation of Pr2ReN4, Nd2ReN4 and Ce2TaN4. Neutron diffraction analysis reveals fully nitrided materials and intricate magnetic structures.
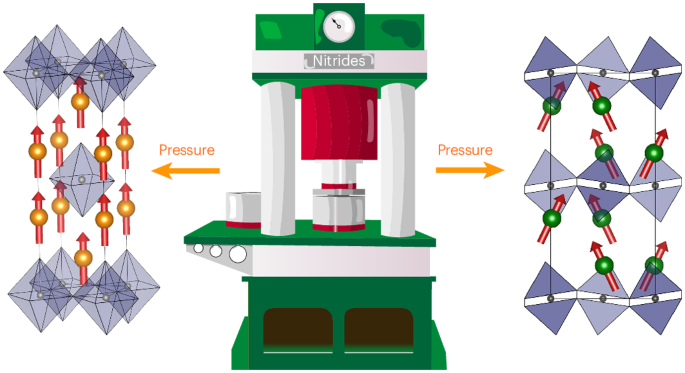
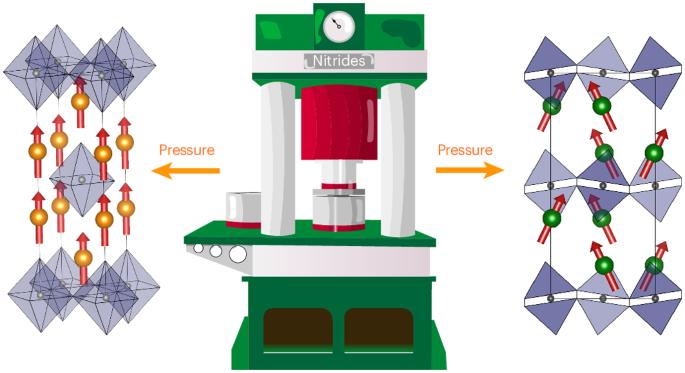
高压合成 Ruddlesden-Popper 氮化物
具有 Ruddlesden-Popper 型结构的层状过氧化物对于低维特性至关重要,例如光伏混合碘化物和超导氧化铜。已知的此类卤化物和氧化物很多,但类似的氮化物却很难稳定,因为需要高阳离子氧化态来平衡阴离子电荷。在此,我们报告了三种单层 Ruddlesden-Popper(K2NiF4 型)氮化物--Pr2ReN4、Nd2ReN4 和 Ce2TaN4 的高压合成及其结构特征和性质。R2ReN4 材料(R = Pr 和 Nd)具有金属性,而 Nd2ReN4 在 15 K 以下具有铁磁性 Nd3+ 自旋阶。Ce2TaN4 的结构转变是由八面体倾斜驱动的,局部畸变和倾斜的磁性 Ce3+ 秩证明了二维 Ce3+/Ce4+ 电荷有序相关性。我们的工作表明,可以通过高压合成制备出具有不同结构、电子和磁性能的 Ruddlesden-Popper 氮化物,从而为相关的层状氮化物材料打开了大门。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature chemistry
化学-化学综合
CiteScore
29.60
自引率
1.40%
发文量
226
审稿时长
1.7 months
期刊介绍:
Nature Chemistry is a monthly journal that publishes groundbreaking and significant research in all areas of chemistry. It covers traditional subjects such as analytical, inorganic, organic, and physical chemistry, as well as a wide range of other topics including catalysis, computational and theoretical chemistry, and environmental chemistry.
The journal also features interdisciplinary research at the interface of chemistry with biology, materials science, nanotechnology, and physics. Manuscripts detailing such multidisciplinary work are encouraged, as long as the central theme pertains to chemistry.
Aside from primary research, Nature Chemistry publishes review articles, news and views, research highlights from other journals, commentaries, book reviews, correspondence, and analysis of the broader chemical landscape. It also addresses crucial issues related to education, funding, policy, intellectual property, and the societal impact of chemistry.
Nature Chemistry is dedicated to ensuring the highest standards of original research through a fair and rigorous review process. It offers authors maximum visibility for their papers, access to a broad readership, exceptional copy editing and production standards, rapid publication, and independence from academic societies and other vested interests.
Overall, Nature Chemistry aims to be the authoritative voice of the global chemical community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


