Probing electrolyte effects on cation-enhanced CO2 reduction on copper in acidic media
IF 42.8
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Tuning the properties of the electric double layer via cations is a recognized approach for improving the efficiency of the CO2 reduction reaction (CO2RR). However, the mechanism behind cation-enhanced CO2RR kinetics remains puzzling. Here we identify the key intermediate, adsorbed CO2, via in situ attenuated total reflection surface-enhanced infrared absorption spectroscopy on Cu in an acidic electrolyte, confirming it appears only in the presence of cations. Different from prevalent viewpoints, time-resolved infrared spectra reveal that Li+ enhances CO2 adsorption more effectively than other larger cations but slows down the hydrogenation kinetics of CO2. Ab initio molecular dynamics simulations and spectroscopic features of water suggest that rigid water networks around Li+ impedes the hydrogen of water to approach adsorbed CO2. In contrast, more flexible water networks around larger cations (for example, Na+) facilitate water reorientation and enhance hydrogen proximity to CO2, thereby improving CO2RR. This study highlights the essential role of interfacial water structure in CO2RR efficiency. CO2 electroreduction is promoted by alkali cations in the electrolyte, but the precise mechanism by which this occurs is not clear. Now in situ infrared spectroscopy and ab initio molecular dynamics are combined to elucidate the specific role of alkali cations and their trends.
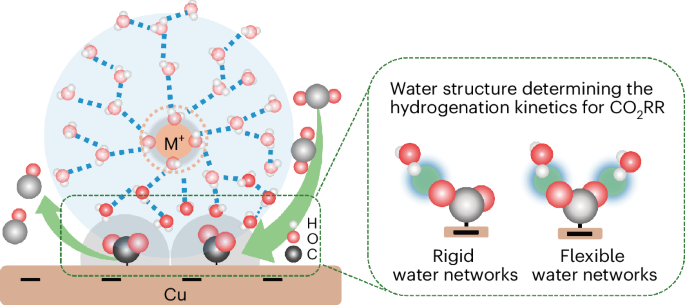
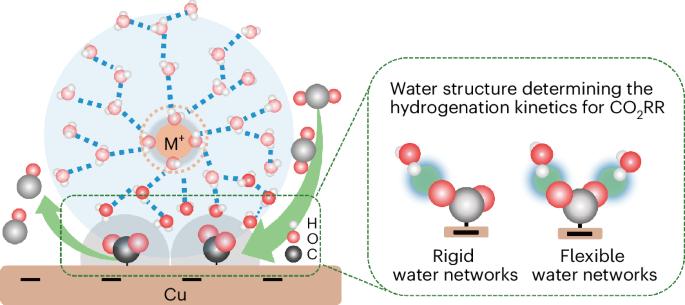
探究电解质对酸性介质中阳离子强化二氧化碳还原铜的影响
通过阳离子调节电双层的特性是一种公认的提高二氧化碳还原反应(CO2RR)效率的方法。然而,阳离子增强 CO2RR 动力学背后的机制仍然令人费解。在这里,我们通过对酸性电解质中的铜进行原位衰减全反射表面增强红外吸收光谱分析,确定了关键的中间产物--吸附的 CO2,并证实它只在阳离子存在时出现。与普遍观点不同的是,时间分辨红外光谱显示 Li+ 比其他较大的阳离子更有效地增强了对二氧化碳的吸附,但却减慢了二氧化碳的氢化动力学。Ab initio 分子动力学模拟和水的光谱特征表明,Li+ 周围的刚性水网络阻碍了水的氢接近被吸附的 CO2。相比之下,大阳离子(如 Na+)周围更柔性的水网络则有利于水的重新定向,增强氢接近 CO2 的能力,从而提高 CO2RR。这项研究强调了界面水结构在 CO2RR 效率中的重要作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Catalysis
Chemical Engineering-Bioengineering
CiteScore
52.10
自引率
1.10%
发文量
140
期刊介绍:
Nature Catalysis serves as a platform for researchers across chemistry and related fields, focusing on homogeneous catalysis, heterogeneous catalysis, and biocatalysts, encompassing both fundamental and applied studies. With a particular emphasis on advancing sustainable industries and processes, the journal provides comprehensive coverage of catalysis research, appealing to scientists, engineers, and researchers in academia and industry.
Maintaining the high standards of the Nature brand, Nature Catalysis boasts a dedicated team of professional editors, rigorous peer-review processes, and swift publication times, ensuring editorial independence and quality. The journal publishes work spanning heterogeneous catalysis, homogeneous catalysis, and biocatalysis, covering areas such as catalytic synthesis, mechanisms, characterization, computational studies, nanoparticle catalysis, electrocatalysis, photocatalysis, environmental catalysis, asymmetric catalysis, and various forms of organocatalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


