Reciprocal sharing of extracellular proteases and extracellular matrix molecules facilitates Bacillus subtilis biofilm formation
IF 2.6
2区 生物学
Q3 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Extracellular proteases are a class of public good that support growth of Bacillus subtilis when nutrients are in a polymeric form. Bacillus subtilis biofilm matrix molecules are another class of public good that are needed for biofilm formation and are prone to exploitation. In this study, we investigated the role of extracellular proteases in B. subtilis biofilm formation and explored interactions between different public good producer strains across various conditions. We confirmed that extracellular proteases support biofilm formation even when glutamic acid provides a freely available nitrogen source. Removal of AprE from the NCIB 3610 secretome adversely affects colony biofilm architecture, while sole induction of WprA activity into an otherwise extracellular protease-free strain is sufficient to promote wrinkle development within the colony biofilm. We found that changing the nutrient source used to support growth affected B. subtilis biofilm structure, hydrophobicity and architecture. We propose that the different phenotypes observed may be due to increased protease dependency for growth when a polymorphic protein presents the sole nitrogen source. We however cannot exclude that the phenotypic changes are due to alternative matrix molecules being made. Co-culture of biofilm matrix and extracellular protease mutants can rescue biofilm structure, yet reliance on extracellular proteases for growth influences population coexistence dynamics. Our findings highlight the intricate interplay between these two classes of public goods, providing insights into microbial social dynamics during biofilm formation across different ecological niches.
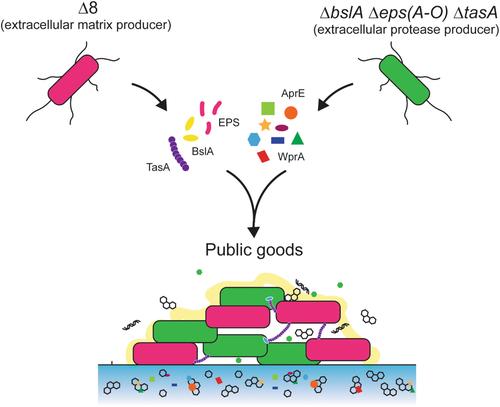
胞外蛋白酶和细胞外基质分子的相互共享有助于枯草杆菌生物膜的形成
胞外蛋白酶是一类公益物,当营养物质以聚合物形式存在时,它能支持枯草芽孢杆菌的生长。枯草芽孢杆菌生物膜基质分子是生物膜形成所需的另一类公益物,容易被利用。在这项研究中,我们研究了胞外蛋白酶在枯草芽孢杆菌生物膜形成过程中的作用,并探讨了不同公益物生产菌株在不同条件下的相互作用。我们证实,即使谷氨酸提供了可自由获得的氮源,胞外蛋白酶也能支持生物膜的形成。从 NCIB 3610 分泌组中去除 AprE 会对菌落生物膜结构产生不利影响,而在不含细胞外蛋白酶的菌株中仅诱导 WprA 活性就足以促进菌落生物膜内皱纹的形成。我们发现,改变用于支持生长的营养源会影响枯草杆菌生物膜的结构、疏水性和构造。我们认为,观察到的不同表型可能是由于当多态蛋白作为唯一氮源时,生长对蛋白酶的依赖性增加。但我们不能排除表型变化是由于制造了替代基质分子所致。生物膜基质和细胞外蛋白酶突变体的共培养可以挽救生物膜结构,但依赖细胞外蛋白酶生长会影响种群共存动态。我们的研究结果突显了这两类公共产品之间错综复杂的相互作用,为了解不同生态位中生物膜形成过程中的微生物社会动态提供了见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Molecular Microbiology
生物-生化与分子生物学
CiteScore
7.20
自引率
5.60%
发文量
132
审稿时长
1.7 months
期刊介绍:
Molecular Microbiology, the leading primary journal in the microbial sciences, publishes molecular studies of Bacteria, Archaea, eukaryotic microorganisms, and their viruses.
Research papers should lead to a deeper understanding of the molecular principles underlying basic physiological processes or mechanisms. Appropriate topics include gene expression and regulation, pathogenicity and virulence, physiology and metabolism, synthesis of macromolecules (proteins, nucleic acids, lipids, polysaccharides, etc), cell biology and subcellular organization, membrane biogenesis and function, traffic and transport, cell-cell communication and signalling pathways, evolution and gene transfer. Articles focused on host responses (cellular or immunological) to pathogens or on microbial ecology should be directed to our sister journals Cellular Microbiology and Environmental Microbiology, respectively.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


