Ligand-functionalized surfaces for chemoselective heterogeneous catalysis
Abstract
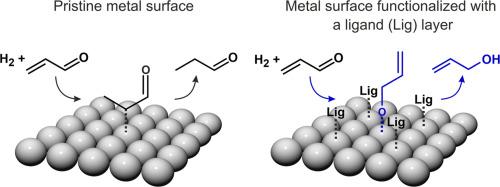
Selectivity of multi-pathway surface reactions depends on subtle differences in the activation barriers of competing reactive processes, which is difficult to control. One of the most promising strategies to overcome this problem is to introduce a specific selective interaction between the reactant and the catalytically active site, directing the chemical transformations towards the desired route. This interaction can be imposed via functionalization of a solid catalyst with organic ligands, promoting the desired pathway via steric constrain and/or electronic effects. The microscopic-level understanding of the underlying surface processes is an important prerequisite for rational design of such new class of ligand-functionalized catalytic materials. In this perspective, we present an overview over our recent mechanistic studies on heterogeneous Pd(111) catalysts functionalized with different types of organic ligands for chemoselective hydrogenation of a,b-unsaturated aldehyde acrolein. Employing a combination of real space microscopic (STM) and in operando spectroscopic (IRAS) surface sensitive techniques, we show that self-ordered active ligand layers are formed under operational conditions and identify their chemical nature and the geometric arrangement on the surface turning over. Deposition of a ligand layer renders Pd highly active and nearly 100 % selective toward propenol formation by promoting acrolein adsorption in a specific adsorption configuration via the O atom of the C = O bond. In this adsorption configuration, acrolein can be hydrogenated first to the desired reaction intermediate propenoxy species followed by formation of the target product propenol. Both the reaction intermediate and the final product propenol as well as their time evolution were identified by IRAS and gas phase analysis via quadrupole mass spectrometry (QMS). Particular focus of these studies was on the role of geometric and electronic effects imposed by specific functional groups purposefully introduced in the ligand layer. Obtained atomistic-level insights into the formation and dynamic evolution of the active ligand layer under operational conditions as well as into the role of geometric vs. electronic effects imposed by the ligand provide important input required for controlling chemoselectivity by purposeful surface functionalization.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


