Multifaceted roles of APOE in Alzheimer disease
IF 28.2
1区 医学
Q1 CLINICAL NEUROLOGY
引用次数: 0
Abstract
For the past three decades, apolipoprotein E (APOE) has been known as the single greatest genetic modulator of sporadic Alzheimer disease (AD) risk, influencing both the average age of onset and the lifetime risk of developing AD. The APOEε4 allele significantly increases AD risk, whereas the ε2 allele is protective relative to the most common ε3 allele. However, large differences in effect size exist across ethnoracial groups that are likely to depend on both global genetic ancestry and local genetic ancestry, as well as gene–environment interactions. Although early studies linked APOE to amyloid-β — one of the two culprit aggregation-prone proteins that define AD — in the past decade, mounting work has associated APOE with other neurodegenerative proteinopathies and broader ageing-related brain changes, such as neuroinflammation, energy metabolism failure, loss of myelin integrity and increased blood–brain barrier permeability, with potential implications for longevity and resilience to pathological protein aggregates. Novel mouse models and other technological advances have also enabled a number of therapeutic approaches aimed at either attenuating the APOEε4-linked increased AD risk or enhancing the APOEε2-linked AD protection. This Review summarizes this progress and highlights areas for future research towards the development of APOE-directed therapeutics. Apolipoprotein E (APOE) is the greatest genetic modulator of sporadic Alzheimer disease risk. This Review provides a comprehensive update on our current knowledge of the genetics of APOE and its role in Alzheimer and other neurodegenerative diseases, and summarizes emerging APOE-targeted therapies designed to prevent or slow down Alzheimer disease.
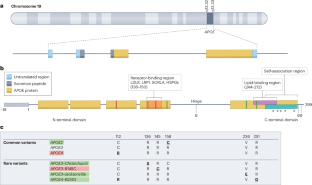
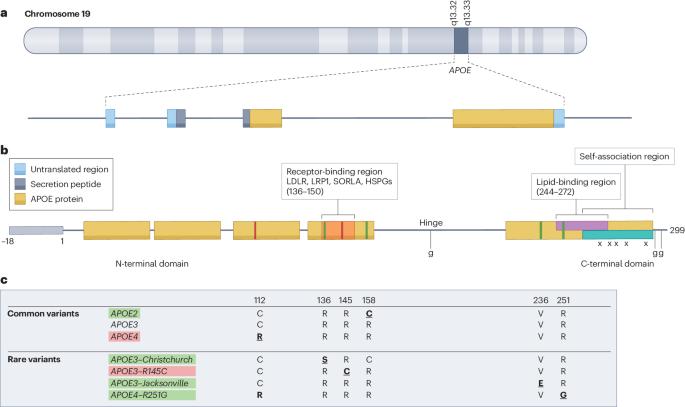
APOE 在阿尔茨海默病中的多方面作用
在过去的三十年中,脂蛋白 E(APOE)一直被认为是散发性阿尔茨海默病(AD)风险的最大遗传调节因素,它既影响发病的平均年龄,也影响罹患 AD 的终生风险。APOEε4 等位基因会显著增加阿尔茨海默病风险,而相对于最常见的ε3 等位基因,ε2 等位基因则具有保护作用。然而,不同人种群体的效应大小存在很大差异,这可能取决于全球遗传血统和当地遗传血统,以及基因与环境的相互作用。虽然早期的研究将 APOE 与淀粉样蛋白-β--确定注意力缺失症的两种易聚集的罪魁祸首蛋白之一联系起来,但在过去十年中,越来越多的研究将 APOE 与其他神经退行性蛋白病和更广泛的与衰老相关的脑部变化联系起来,如神经炎症、能量代谢衰竭、髓鞘完整性丧失和血脑屏障通透性增加,这对人的寿命和对病理蛋白聚集的恢复能力具有潜在的影响。新颖的小鼠模型和其他技术进步也促成了许多治疗方法,这些方法旨在减弱与 APOEε4 相关的注意力缺失症风险增加或增强与 APOEε2 相关的注意力缺失症保护。本综述总结了这一进展,并强调了未来开发 APOE 定向疗法的研究领域。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Reviews Neurology
医学-临床神经学
CiteScore
29.90
自引率
0.80%
发文量
138
审稿时长
6-12 weeks
期刊介绍:
Nature Reviews Neurology aims to be the premier source of reviews and commentaries for the scientific and clinical communities we serve. We want to provide an unparalleled service to authors, referees, and readers, and we work hard to maximize the usefulness and impact of each article. The journal publishes Research Highlights, Comments, News & Views, Reviews, Consensus Statements, and Perspectives relevant to researchers and clinicians working in the field of neurology. Our broad scope ensures that the work we publish reaches the widest possible audience. Our articles are authoritative, accessible, and enhanced with clearly understandable figures, tables, and other display items. This page gives more detail about the aims and scope of the journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


