A novel homozygous variant of the PIGK gene caused by paternal disomy in a patient with neurodevelopmental disorder, cerebellar atrophy, and seizures
IF 2.6
3区 生物学
Q2 GENETICS & HEREDITY
引用次数: 0
Abstract
Glycosylphosphatidylinositol (GPI)-anchored proteins are located at the cell surface by a covalent attachment between protein and GPI embedded in the plasma membrane. This attachment is catalyzed by GPI transamidase comprising five subunits (PIGK, PIGS, PIGT, PIGU, and GPAA1) in the endoplasmic reticulum. Loss of either subunit of GPI transamidase eliminates cell surface localization of GPI-anchored proteins. In humans, pathogenic variants in either subunit of GPI transamidase cause neurodevelopmental disorders. However, how the loss of GPI-anchored proteins triggers neurodevelopmental defects remains largely unclear. Here, we identified a novel homozygous variant of PIGK, NM_005482:c.481A > G,p. (Met161Val), in a Japanese female patient with neurodevelopmental delay, hypotonia, cerebellar atrophy, febrile seizures, hearing loss, growth impairment, dysmorphic facial features, and brachydactyly. The missense variant was found heterozygous in her father, but not in her mother. Zygosity analysis revealed that the homozygous PIGK variant in the patient was caused by paternal isodisomy. Rescue experiments using PIGK-deficient CHO cells revealed that the p.Met161Val variant of PIGK reduced GPI transamidase activity. Rescue experiments using pigk mutant zebrafish confirmed that the p.Met161Val variant compromised PIGK function in tactile-evoked motor response. We also demonstrated that axonal localization of voltage-gated sodium channels and concomitant generation of action potentials were impaired in pigk-deficient neurons in zebrafish, suggesting a link between GPI-anchored proteins and neuronal defects. Taken together, the missense p.Met161Val variant of PIGK is a novel pathogenic variant that causes the neurodevelopmental disorder.
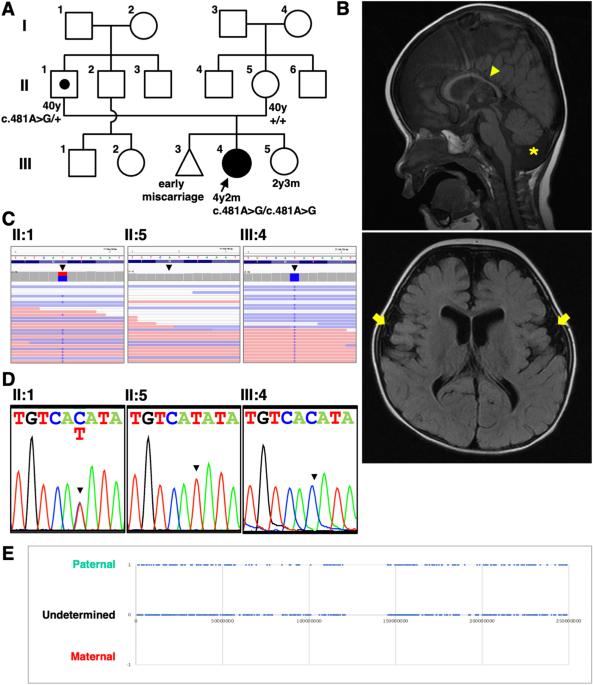
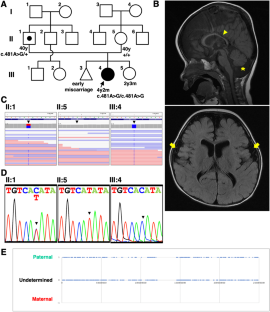
一名患有神经发育障碍、小脑萎缩和癫痫发作的患者因父系断裂而导致的 PIGK 基因新型同卵变体。
糖基磷脂酰肌醇(GPI)锚定蛋白质是通过蛋白质与嵌入质膜的 GPI 之间的共价连接而位于细胞表面的。这种附着是由内质网中由五个亚基(PIGK、PIGS、PIGT、PIGU 和 GPAA1)组成的 GPI 转酰胺酶催化的。GPI 转酰胺酶任一亚基的缺失都会消除 GPI 锚定蛋白在细胞表面的定位。在人类中,GPI 转酰胺酶任一亚基的致病变体都会导致神经发育障碍。然而,GPI锚定蛋白的缺失是如何引发神经发育缺陷的,目前仍不清楚。在这里,我们发现了一种新的 PIGK 同源变体 NM_005482:c.481A>G,p.(Met161Val),该患者为日本女性,患有神经发育迟缓、肌张力低下、小脑萎缩、发热性癫痫、听力损失、生长障碍、面部畸形和腕骨发育不良。该错义变异在她的父亲中是杂合的,但在她的母亲中没有发现。显性遗传分析表明,该患者的同源 PIGK 变体是由父系同位异体切除术引起的。利用PIGK缺陷的CHO细胞进行的拯救实验发现,PIGK的p.Met161Val变体降低了GPI转氨酶的活性。利用pigk突变斑马鱼进行的拯救实验证实,p.Met161Val变体损害了PIGK在触觉诱发的运动反应中的功能。我们还证明,电压门控钠通道的轴突定位以及随之产生的动作电位在pigk缺陷斑马鱼神经元中受损,这表明GPI锚定蛋白与神经元缺陷之间存在联系。综上所述,PIGK的错义p.Met161Val变体是一种导致神经发育障碍的新型致病变体。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Human Genetics
生物-遗传学
CiteScore
7.20
自引率
0.00%
发文量
101
审稿时长
4-8 weeks
期刊介绍:
The Journal of Human Genetics is an international journal publishing articles on human genetics, including medical genetics and human genome analysis. It covers all aspects of human genetics, including molecular genetics, clinical genetics, behavioral genetics, immunogenetics, pharmacogenomics, population genetics, functional genomics, epigenetics, genetic counseling and gene therapy.
Articles on the following areas are especially welcome: genetic factors of monogenic and complex disorders, genome-wide association studies, genetic epidemiology, cancer genetics, personal genomics, genotype-phenotype relationships and genome diversity.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


