Catalytic Olefin Transpositions Facilitated by Ruthenium N,N,N-Pincer Complexes
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
In this report, we demonstrate olefin transposition/isomerization reactions catalyzed by a series of N,N,N-pincer (1,3-bis(2-pyridylimino)isoindoline) Ru-hydride complexes. The protocol proceeds at room temperature for most substrates, achieving excellent yields, regioselectivity, and diastereoselectivity in short reaction times. The air-stable Ru-chloride derivatives of these complexes exhibit comparable reactivity enabling benchtop setup and synthetic versatility. Furthermore, we demonstrate the potential for one-pot cascade sequences of the products derived from the transposition reactions.
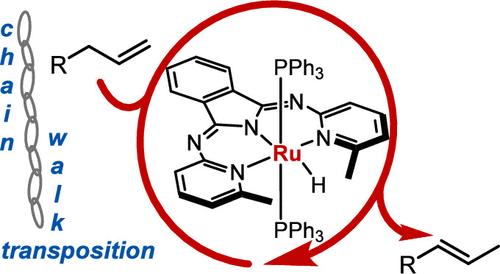
N,N,N-钌钳配合物催化烯烃转化。
在本报告中,我们展示了一系列 N,N,N-incer(1,3-双(2-吡啶亚氨基)异吲哚啉)Ru-酸酐配合物催化的烯烃转位/异构化反应。对于大多数底物,该方案都能在室温下进行,并能在较短的反应时间内获得优异的产率、区域选择性和非对映选择性。这些复合物在空气中稳定的 Ru Chloride 衍生物具有可比的反应活性,实现了台式设置和合成的多功能性。此外,我们还展示了转位反应衍生产物的单锅级联序的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
The Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


