Thermogravimetric analysis on two Bambusa texlitis wastes slow pyrolysis behaviors and kinetics using isoconversional and parallel-reactions models
Abstract
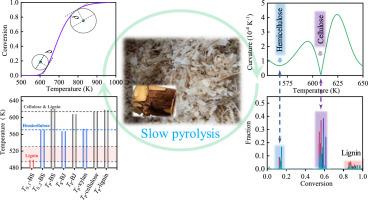
In this work, the pyrolysis behaviors and kinetics of two bamboo (Bambusa texlitis) wastes were investigated via thermogravimetric analysis. The weight loss behaviors of pyrolysis were studied by curvature of the conversion (α) curves. The curvature of α can depict the characteristic temperatures of pseudo component (hemicellulose, cellulose and lignin) pyrolysis. Model-free model was applied to estimate the apparent activation energies of the pure components for comparison with other kinetic models. Three parallel reaction (TPR) and parallel finite number first-order reaction (FNFOR) models were used for kinetic studies. The first-order TPR model underestimates the apparent activation energies of pseudo components. The nth-order TPR model underestimates the apparent activation energy of lignin and obtains characteristic temperatures that are inconsistent with those of pure lignin. The parallel FNFOR model is more reliable for simulating the kinetics of lignocellulosic biomass pyrolysis. The apparent activation energies of pseudo components depend on the variety of lignocellulosic biomass.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


