Nanoparticle-mediated celastrol ER targeting delivery amplify immunogenic cell death in melanoma
IF 11.4
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
Introduction
Chemoimmunotherapy, which benefits from the combination of chemotherapy and immunotherapy, has emerged as a promising strategy in cancer treatment. However, effectively inducing a robust immune response remains challenging due to the limited responsiveness across patients. Endoplasmic reticulum (ER) stress is essential for activating intracellular signaling pathways associated with immunogenic cell death (ICD), targeting drugs to ER might enhance ER stress and improve ICD-related immunotherapy.
Objectives
To improve the immune response of Chemoimmunotherapy.
Methods
ER targeting nanoparticles TSE-CEL/NP were constructed to enhance immunogenic cancer cell death. Flow cytometry, confocal microscope, TEM and immunofluorescence were used to evaluate the ER targeting effect and immunogenic tumor cell death in vitro on B16F10 tumor cells. Unilateral and bilateral tumor models were constructed to investigate the efficacy of anti-tumor and immunotherapy in vivo. Lung metastasis B16F10 melanoma tumor-bearing mice were used to assess the anti-metastasis efficacy.
Results
TSE-CEL/NP could specially accumulate in ER, thereby induce ER stress. High ER stress trigger the exposure of CRT, the extracellular release of HMGB1 and ATP. These danger signals subsequently promote the recruitment and maturation of dendritic cells (DCs), which in turn increase the proliferation of cytotoxic T lymphocytes (CD8+ T cells), ultimately resulted in an improved immunotherapy efficacy against melanoma. In vivo experiments showed that TSE-CEL/NP exhibits excellent antitumor efficacy and triggers a strong immune response.
Conclusion
Our findings demonstrated that celastrol ER targeting delivery could amplify immunogenic cell death in melanoma, which provide experimental basis for melanoma immunotherapy.
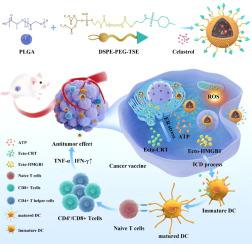

纳米颗粒介导的 celastrol ER 靶向递送可扩大黑色素瘤的免疫细胞死亡。
简介:化疗免疫疗法得益于化疗和免疫疗法的结合:化疗免疫疗法得益于化疗和免疫疗法的结合,已成为癌症治疗中一种前景广阔的策略。然而,由于不同患者的反应能力有限,有效诱导强有力的免疫反应仍具有挑战性。内质网(ER)应激对于激活与免疫原性细胞死亡(ICD)相关的细胞内信号通路至关重要,靶向ER的药物可能会增强ER应激,改善与ICD相关的免疫疗法:改善化疗免疫疗法的免疫反应:方法:构建ER靶向纳米粒子TSE-CEL/NP,以增强免疫性癌细胞死亡。采用流式细胞术、共聚焦显微镜、TEM和免疫荧光技术评估ER靶向效应和B16F10肿瘤细胞体外免疫原性肿瘤细胞死亡。建立单侧和双侧肿瘤模型,研究体内抗肿瘤和免疫治疗的疗效。用肺转移B16F10黑色素瘤小鼠评估抗转移疗效:结果:TSE-CEL/NP能在ER中特殊积累,从而诱导ER应激。高ER应激会触发CRT暴露、细胞外释放HMGB1和ATP。这些危险信号随后会促进树突状细胞(DC)的募集和成熟,进而增加细胞毒性 T 淋巴细胞(CD8+ T 细胞)的增殖,最终提高黑色素瘤免疫疗法的疗效。体内实验表明,TSE-CEL/NP 具有出色的抗肿瘤疗效,并能引发强烈的免疫反应:我们的研究结果为黑色素瘤免疫疗法提供了一种新的ER靶向方法和实验基础。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Advanced Research
Multidisciplinary-Multidisciplinary
CiteScore
21.60
自引率
0.90%
发文量
280
审稿时长
12 weeks
期刊介绍:
Journal of Advanced Research (J. Adv. Res.) is an applied/natural sciences, peer-reviewed journal that focuses on interdisciplinary research. The journal aims to contribute to applied research and knowledge worldwide through the publication of original and high-quality research articles in the fields of Medicine, Pharmaceutical Sciences, Dentistry, Physical Therapy, Veterinary Medicine, and Basic and Biological Sciences.
The following abstracting and indexing services cover the Journal of Advanced Research: PubMed/Medline, Essential Science Indicators, Web of Science, Scopus, PubMed Central, PubMed, Science Citation Index Expanded, Directory of Open Access Journals (DOAJ), and INSPEC.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


