Discovery of isoquinoline sulfonamides as allosteric gyrase inhibitors with activity against fluoroquinolone-resistant bacteria
IF 19.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Bacteria have evolved resistance to nearly all known antibacterials, emphasizing the need to identify antibiotics that operate via novel mechanisms. Here we report a class of allosteric inhibitors of DNA gyrase with antibacterial activity against fluoroquinolone-resistant clinical isolates of Escherichia coli. Screening of a small-molecule library revealed an initial isoquinoline sulfonamide hit, which was optimized via medicinal chemistry efforts to afford the more potent antibacterial LEI-800. Target identification studies, including whole-genome sequencing of in vitro selected mutants with resistance to isoquinoline sulfonamides, unanimously pointed to the DNA gyrase complex, an essential bacterial topoisomerase and an established antibacterial target. Using single-particle cryogenic electron microscopy, we determined the structure of the gyrase–LEI-800–DNA complex. The compound occupies an allosteric, hydrophobic pocket in the GyrA subunit and has a mode of action that is distinct from the clinically used fluoroquinolones or any other gyrase inhibitor reported to date. LEI-800 provides a chemotype suitable for development to counter the increasingly widespread bacterial resistance to fluoroquinolones. Global antibiotic scarcity looms owing to bacterial resistance. Now the discovery of a class of allosteric inhibitors targeting DNA gyrase—essential for bacteria—yields a compound LEI-800 that exhibits activity against fluoroquinolone-resistant E. coli. The compound’s unique mode of action, revealed through cryo-EM, makes it a promising candidate for countering bacterial resistance.
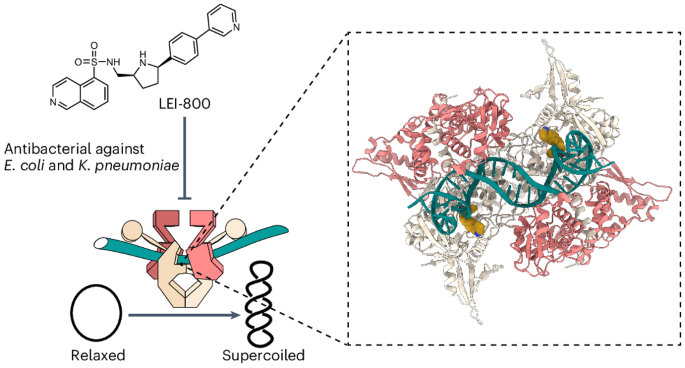
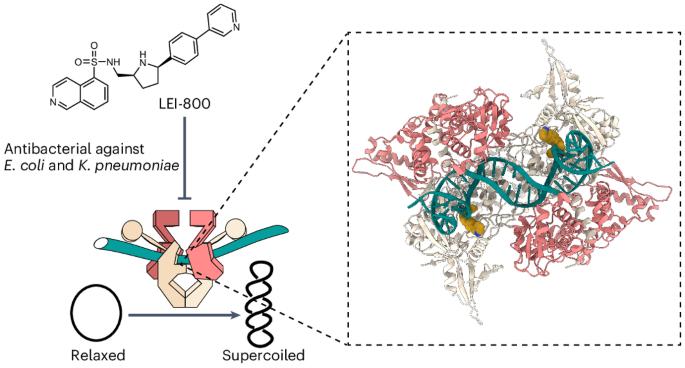
发现异喹啉磺酰胺类异位回旋酶抑制剂,具有抗氟喹诺酮耐药细菌的活性
细菌已进化出对几乎所有已知抗菌药的耐药性,因此需要找出通过新机制发挥作用的抗生素。我们在此报告了一类 DNA 回旋酶异位抑制剂,它们对耐氟喹诺酮的临床大肠杆菌分离株具有抗菌活性。通过筛选小分子化合物库,我们发现了一个最初的异喹啉磺酰胺类药物,并通过药物化学的努力对其进行了优化,从而得到了更强效的抗菌药 LEI-800。目标识别研究(包括对体外筛选出的对异喹啉磺胺类药物具有抗药性的突变体进行全基因组测序)一致指向了 DNA 回旋酶复合物,这是一种重要的细菌拓扑异构酶,也是一个公认的抗菌目标。我们利用单颗粒低温电子显微镜测定了回旋酶-LEI-800-DNA 复合物的结构。该化合物占据了 GyrA 亚基中的一个异位疏水口袋,其作用模式有别于临床使用的氟喹诺酮类药物或迄今报道的任何其他回旋酶抑制剂。LEI-800 提供了一种适合开发的化学类型,以应对细菌对氟喹诺酮类药物日益广泛的耐药性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature chemistry
化学-化学综合
CiteScore
29.60
自引率
1.40%
发文量
226
审稿时长
1.7 months
期刊介绍:
Nature Chemistry is a monthly journal that publishes groundbreaking and significant research in all areas of chemistry. It covers traditional subjects such as analytical, inorganic, organic, and physical chemistry, as well as a wide range of other topics including catalysis, computational and theoretical chemistry, and environmental chemistry.
The journal also features interdisciplinary research at the interface of chemistry with biology, materials science, nanotechnology, and physics. Manuscripts detailing such multidisciplinary work are encouraged, as long as the central theme pertains to chemistry.
Aside from primary research, Nature Chemistry publishes review articles, news and views, research highlights from other journals, commentaries, book reviews, correspondence, and analysis of the broader chemical landscape. It also addresses crucial issues related to education, funding, policy, intellectual property, and the societal impact of chemistry.
Nature Chemistry is dedicated to ensuring the highest standards of original research through a fair and rigorous review process. It offers authors maximum visibility for their papers, access to a broad readership, exceptional copy editing and production standards, rapid publication, and independence from academic societies and other vested interests.
Overall, Nature Chemistry aims to be the authoritative voice of the global chemical community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


